Changes Around Us: Physical and Chemical Class 7 Notes Science Chapter 5 Free PDF
Introduction
We see many changes happening all around us every day. Ice melts into water, flowers bloom from buds, fruits change color and smell, and cold water becomes warm over time. These changes can affect how things look, smell, feel, or taste. Using our five senses — sight, smell, touch, hearing, and taste — we can observe and understand these changes better. 
Let’s explore some common changes around us and learn how to notice them carefully.
Physical Change Vs Chemical Change
Physical Change
A physical change is when a substance or object changes in appearance (e.g., shape, size, state) but remains the same substance, with no new material formed.
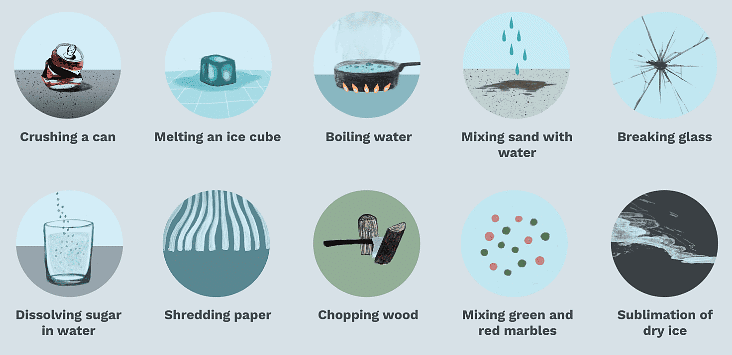 Physical Changes
Physical Changes
Key Features
- Only physical properties like shape, size, or state change.
- The substance’s chemical composition remains unchanged.
- Examples include melting ice, boiling water, or folding clothes.
Examples
- Paper Folding: Folding paper into shapes (e.g., a boat or plane) changes its form, but unfolding it returns the original paper.

- Balloon Inflation: Inflating a balloon stretches it, but letting the air out returns it to its original shape. However, pricking an inflated balloon causes it to burst, and the original shape cannot be restored, though the rubber material remains the same.
- Crushing Chalk: Crushing chalk into powder changes its form, but the material (chalk) stays the same, though the original piece cannot be reformed.

- Water’s States: Water changing from solid (ice) to liquid (water) to gas (steam) is a physical change, as it remains water despite changing states.
Chemical Change
When substances react chemically, they form new substances with different properties. This is called a chemical change. Unlike physical changes where only appearance or state changes, chemical changes create entirely new materials.
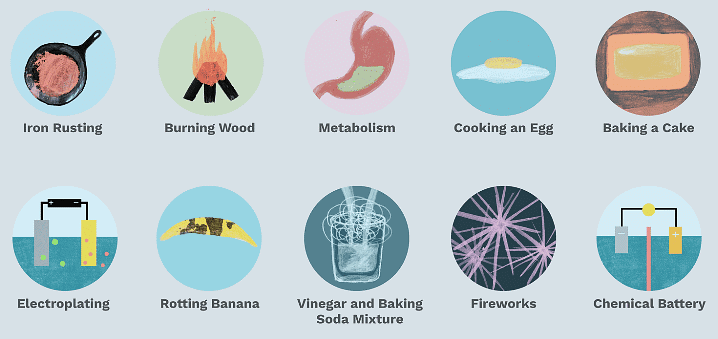 Chemical ChangesExamples:
Chemical ChangesExamples:
1. Blowing Air into Lime Water
- A common example is when carbon dioxide (CO₂) gas comes into contact with lime water (a solution of calcium hydroxide).
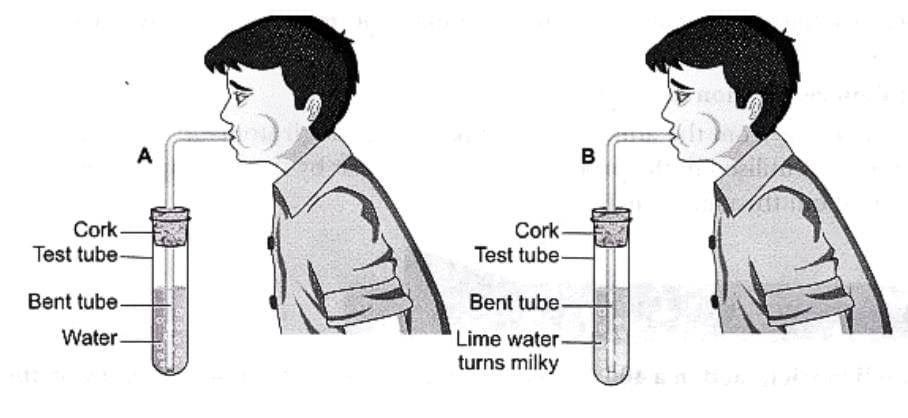 Lime Water Turns Milky
Lime Water Turns Milky - The carbon dioxide reacts with the lime water to form calcium carbonate, which is a white solid that makes the solution look milky or cloudy.
- This solid eventually settles at the bottom of the container.
- Along with calcium carbonate, water is also formed during this reaction.
- This reaction can be written as:
Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - This shows that a new substance, calcium carbonate, is produced, proving that a chemical change has taken place.
2. Vinegar and Baking Soda
- Another example is the reaction between vinegar (which contains acetic acid) and baking soda (sodium bicarbonate).

- When these two substances mix, they react to produce carbon dioxide gas, which can be seen as bubbling or fizzing.
- This gas, when passed through lime water, turns the lime water milky due to the formation of calcium carbonate.
- This confirms that carbon dioxide gas was produced by the chemical reaction.
Some Other Processes Involving Chemical Changes
1. Rusting
- Rusting is a chemical change where iron reacts with air and moisture to form a new substance called rust (iron oxide).
- This brown-colored substance forms on iron objects like nails and makes them weak and crumbly.
- Since a new substance is formed, rusting is a chemical change.
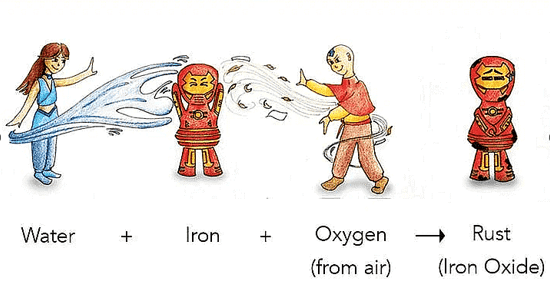 Rusting of Iron
Rusting of Iron
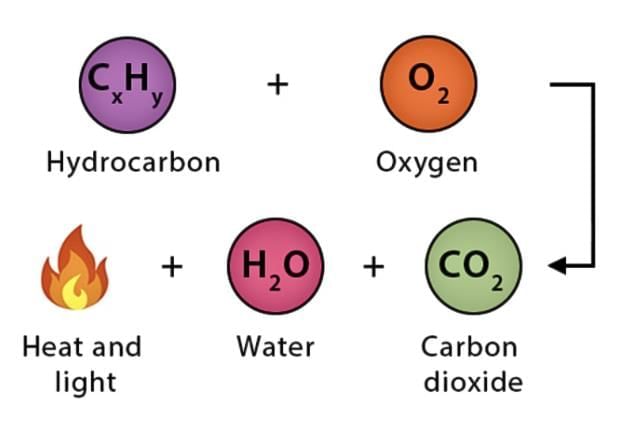
2. Combustion
Combustion is a chemical reaction where a substance reacts with oxygen, producing heat and/or light. Substances that burn are called combustible substances (e.g., wood, paper, cotton, kerosene).
- An example is burning magnesium ribbon. When magnesium burns in air, it forms magnesium oxide, a white powder, and releases heat and light.
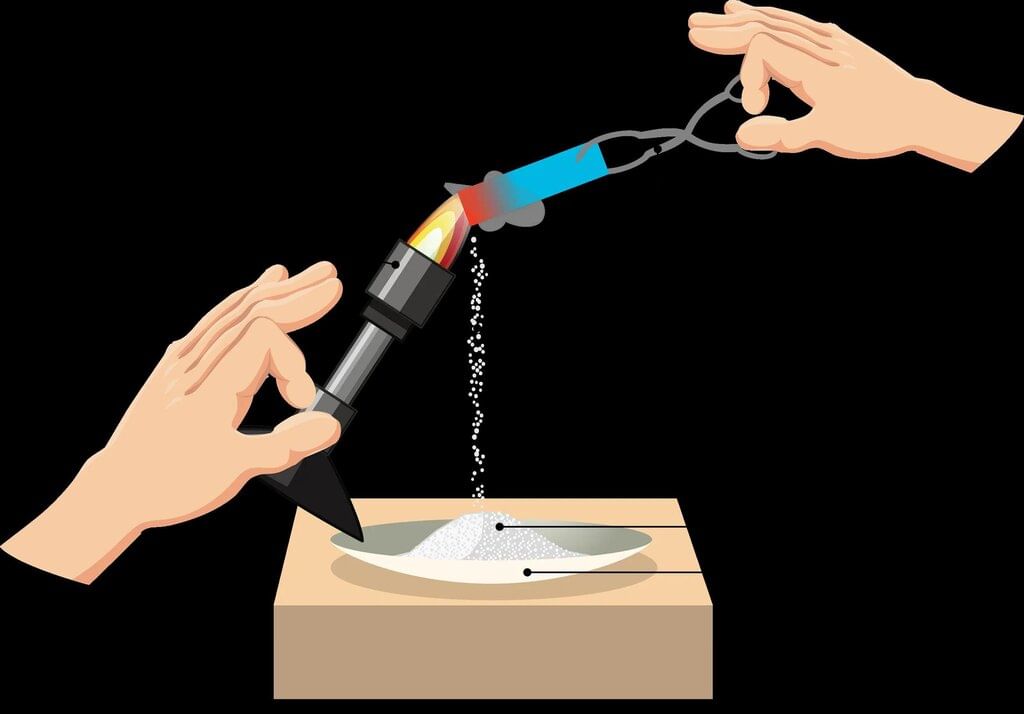
- This shows that combustion involves forming new substances along with energy release.
- The chemical reaction for burning magnesium can be written as:
Magnesium + Oxygen → Magnesium oxide + Heat + Light
(Ribbon) (Air) (White powder) - Substances that can catch fire and burn are called combustible substances. Examples include wood, paper, cotton, and kerosene.
3. Oxygen’s Role in Combustion
- Oxygen from the air is essential for combustion.
- If you cover a burning candle so it cannot get air, the flame goes out because there is no oxygen to support burning.
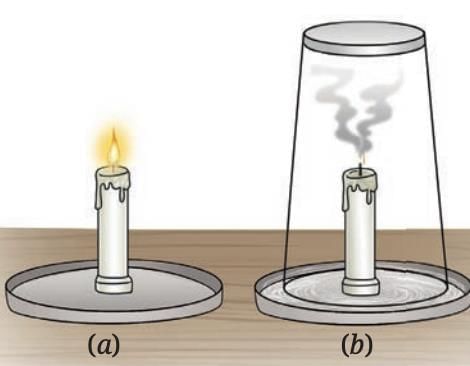 Candle (a) burning (b) covered with a glass tumbler
Candle (a) burning (b) covered with a glass tumbler - During combustion, carbon in the fuel reacts with oxygen to form carbon dioxide gas.
- Carbon dioxide can be detected by passing it through lime water, which turns milky due to the formation of calcium carbonate.
- This confirms that oxygen supports burning and that combustion produces carbon dioxide.
What Else is Needed to Start Combustion?
- Besides fuel (the combustible substance) and oxygen, heat is required to start combustion. This heat raises the fuel’s temperature to a point called the ignition temperature, at which it catches fire.

- For example, paper will catch fire quickly if touched with a lighted matchstick because the matchstick’s temperature is above paper’s ignition temperature.
- However, paper can also catch fire without a matchstick if focused sunlight heats it enough. Using a magnifying glass to concentrate sunlight on paper can increase its temperature until it starts burning.
 Paper catching fire
Paper catching fire
Three Requirements for Combustion
To start and maintain combustion, these three things are necessary:
Fuel (Combustible substance) — Something that can burn.
Oxygen — To support the burning process.
Heat — To raise the fuel to its ignition temperature.
Fire Triangle: Combustion requires fuel, oxygen, and heat (ignition temperature), represented as a triangle.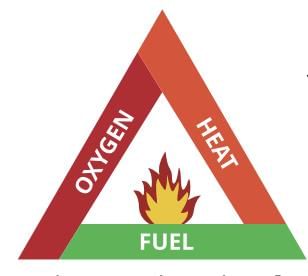 Fire triangle
Fire triangle
Fascinating Fact
Have you seen tiny glowing insects in gardens at night?
They are called fireflies, and their light comes from a chemical change inside their bodies.
This special kind of light, made without heat, is called bioluminescence.
Science and Society
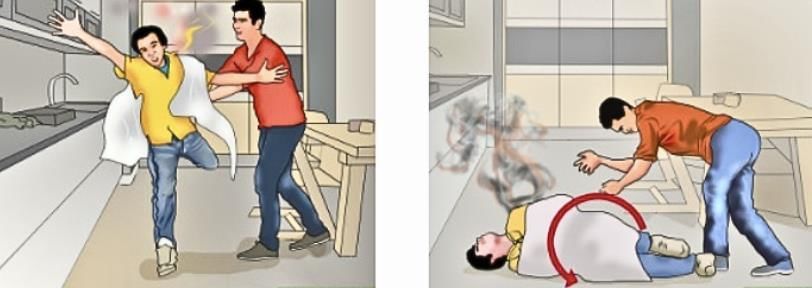
What to do if someone’s clothes catch fire?
Wrap them in a blanket or cloth to stop the air supply — this helps put out the fire.Important: Never use synthetic blankets or clothes, as they can melt and stick to the skin, causing more harm.
Can Physical and Chemical Changes Occur in the Same Process?
1. Burning a Candle Physical Changes
- Melting Wax: Heat from the flame melts solid wax into liquid, a physical change as no new substance forms.
- Evaporation: Liquid wax moves up the wick and evaporates into vapor, another physical change.
- Solidification: Wax dripping and cooling back into solid is also physical.
2. Burning a Candle Chemical Change:
- Burning Wax Vapor: The wax vapor burns in the presence of oxygen, producing carbon dioxide, water, heat, and light, forming new substances, indicating a chemical change.
Conclusion
Burning a candle involves both physical changes (melting, evaporation) and a chemical change (burning), showing that some processes combine both types.
Know A Scientist: Michael Faraday
Scientist Michael Faraday studied candles in the 19th century, using them to explain physical and chemical processes like melting, vaporization, and combustion in his lectures, “Chemical History of a Candle.”
Are Changes Permanent?
Reversible Changes
Definition: Changes where the original substance or object can be restored.
Examples:
- Melting Ice: Ice melts into water, but freezing the water reforms ice.
- Boiling Water: Water turns to steam, but condensing the steam returns liquid water.
- Folding Paper: Folding and unfolding paper restores its original shape.
Irreversible Changes
Definition: Changes where the original substance or object cannot be restored.
Examples:
- Chopping Vegetables: Cut pieces cannot be reassembled into the whole vegetable.
- Making Popcorn: Corn kernels transform into popcorn, which cannot revert to kernels.
- Burning Wood: Wood turns to ash, which cannot become wood again.
Are All Changes Desirable?
Desirable Changes
Changes that are useful or beneficial.
Examples:
- Milk to Curd: Curdling produces tasty curd.
- Ripening Fruits: Fruits become sweet and edible.
- Cooking Food: Raw ingredients become nutritious meals.
- Cutting Fruits: Makes them easier to eat.
Undesirable Changes
Changes that are harmful or unwanted.
Examples:
- Rusting of Iron: Damages tools and structures.
- Food Decay: Spoils stored food, making it inedible.
Some changes can be good or bad depending on the situation
Decomposing food is bad if it rots in the kitchen, but good when it’s used to make compost for plants.
Environmental Impact
- Human activities, like burning fuels in vehicles or drying paint, release carbon dioxide and pollutants, increasing atmospheric pollution over time.
- These changes have long-term effects on the environment, like climate change.

Some Slow Natural Changes
Weathering of Rocks
Weathering is the process where rocks break down into smaller pieces (sediments) through physical and chemical changes, eventually forming soil.
1. Physical Weathering
- Caused by temperature changes, tree roots growing into cracks, or water freezing in rock crevices, breaking rocks into smaller pieces.
- Example: Sediments like sand, soil, and stones collect at the base of mountains or cliffs.
2. Chemical Weathering:
- Occurs when water or chemicals in water react with rock components.
- Example: Basalt rock, containing iron, reacts with water or air to form a red layer of iron oxide (similar to rust), changing its composition.
Together, these changes help break down rocks and form soil, which is important for plant growth and life on Earth.
Erosion
Erosion is the physical process where rocks, soil, and sediments are broken down and moved by natural forces like wind or flowing water.
Examples
You may have seen fine sand on riverbeds or lakes—this is created when larger rocks and soil are worn down and moved by rivers or wind.
River rocks become smooth over time because the flowing water keeps rubbing them, slowly wearing them down.
During landslides, erosion happens quickly, as large amounts of soil and rock are displaced. This is an example of a physical change.
When wind or water slows down (like in lakes or oceans), the carried sediments settle at the bottom.
Over thousands of years, these sediments get pressed and hardened to form new rocks.
These changes are slow, natural, and usually irreversible.
Points to Remember
Physical changes: Only shape, size, or state changes. No new substance is formed.
Examples: Melting ice, boiling water, folding clothes.Chemical changes: New substances are formed.
Examples: Rusting, burning, lime water turning milky with carbon dioxide.Rusting: Iron reacts with air and water to form rust — a new substance.
Combustion: Burning with oxygen produces heat and light. Needs fuel, oxygen, and enough heat (ignition temperature).
Fireflies: Glow due to a chemical change called bioluminescence, which gives light without heat.
Burning a candle:
Physical change: Wax melts and evaporates.
Chemical change: Wax vapour burns to form carbon dioxide and water.
Reversible changes: Can go back (e.g., melting ice).
Irreversible changes: Cannot go back (e.g., chopping vegetables).Desirable changes: Helpful (e.g., cooking food, making curd).
Undesirable changes: Harmful (e.g., rusting, food spoilage), but some like decomposition can help make compost.Human activities: Burning fuel and drying paint release gases that pollute the air and increase carbon dioxide.
Weathering: Rocks break into soil slowly due to temperature or water — both physical and chemical changes.
Erosion: Wind or water moves rocks and soil. It shapes the land but is often irreversible.
Michael Faraday: A scientist who used candles to explain physical and chemical changes in simple ways.
Difficult Words and Their Meanings
- Physical Change: A change in a substance’s appearance (e.g., shape, size, state) without forming a new substance, like melting ice.
- Chemical Change: A change where new substances are formed through a chemical reaction, like rusting or burning.
- Chemical Reaction: A process where substances (reactants) transform into new substances (products), e.g., vinegar and baking soda forming carbon dioxide.
- Rusting: A chemical change where iron reacts with air and water to form brown rust (iron oxide).
- Combustion: A chemical reaction where a substance burns with oxygen, producing heat and/or light, e.g., burning magnesium.
- Combustible Substance: A material that can burn, like wood, paper, or kerosene.
- Ignition Temperature: The minimum temperature at which a substance catches fire, e.g., paper’s ignition temperature when heated by a matchstick.
- Fire Triangle: The three requirements for combustion—fuel, oxygen, and heat (ignition temperature).
- Reversible Change: A change where the original substance can be restored, e.g., freezing melted water.
- Irreversible Change: A change where the original substance cannot be restored, e.g., burning wood to ash.
- Desirable Change: A beneficial change, like cooking food or ripening fruits.
- Undesirable Change: A harmful change, like rusting iron or food spoilage.
- Weathering: The physical and chemical breakdown of rocks into soil, caused by temperature, water, or chemicals.
- Erosion: The physical movement of rocks, soil, or sediments by wind or water, shaping landscapes.
- Bioluminescence: Light produced by living organisms, like fireflies, through a chemical change without heat.
|
80 videos|319 docs|12 tests
|
FAQs on Changes Around Us: Physical and Chemical Class 7 Notes Science Chapter 5 Free PDF
| 1. What are physical changes and how do they differ from chemical changes? |  |
| 2. Can you provide examples of everyday chemical changes? |  |
| 3. How can we identify a chemical change has occurred? |  |
| 4. What role do catalysts play in chemical reactions? |  |
| 5. Why is it important to understand the differences between physical and chemical changes? |  |