Class 7 Science Chapter 5 Worksheet Solutions - Changes Around Us: Physical and Chemical
Q.1. True/False
(i) Rusting of iron is a physical change.
False
Explanation: It is a chemical change where new substance (rust) is formed.
(ii) Evaporation of water is a reversible change.
True
Explanation: Water vapor can condense back into liquid.
(iii) All physical changes are reversible.
False
Explanation: Some physical changes, like chopping wood, are irreversible.
(iv) Burning of magnesium ribbon produces a white powder.
True
Explanation: It forms magnesium oxide, a new substance.
(v) Bioluminescence produces light without heat.
True
Explanation: It is a chemical change found in fireflies.
Q.2. Fill in the blanks.
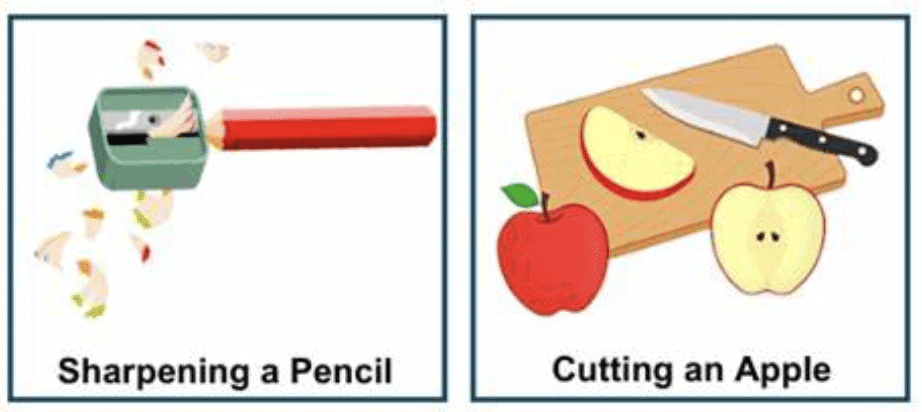
(i) Cutting a log of wood into pieces is a ____ change.
physical
This is a physical change because it alters the form of the wood without changing its chemical composition.
(ii) Condensation is a ____ change.
physical
Condensation is a physical change as it involves a change in the state of matter from gas to liquid without altering the substance's chemical identity.
(iii) When carbon dioxide is passed through lime water, it turns milky due to the formation of ____.
Calcium Carbonate
The milky appearance is due to the formation of calcium carbonate, which occurs when carbon dioxide reacts with lime water.
(iv) Two methods by which rusting of iron can be prevented are ____ and ____.
painting or greasing, galvanisation
Painting or greasing creates a barrier against moisture, while galvanisation involves coating iron with zinc to prevent rust.
(v) Changes in which only ____ properties of a substance change are called physical changes.
physical
Physical changes involve alterations in physical properties such as shape, size, or state, without changing the substance's chemical identity.
(vi) A medicine is the end product of a chain of ____.
chemical reactions
Medicines are created through a series of chemical reactions that transform raw materials into effective therapeutic agents.
Answer the following Questions
Q3. What is the white substance formed when lime water reacts with carbon dioxide?
Calcium carbonate.
Q4. What kind of change is chopping vegetables?
Irreversible physical change.
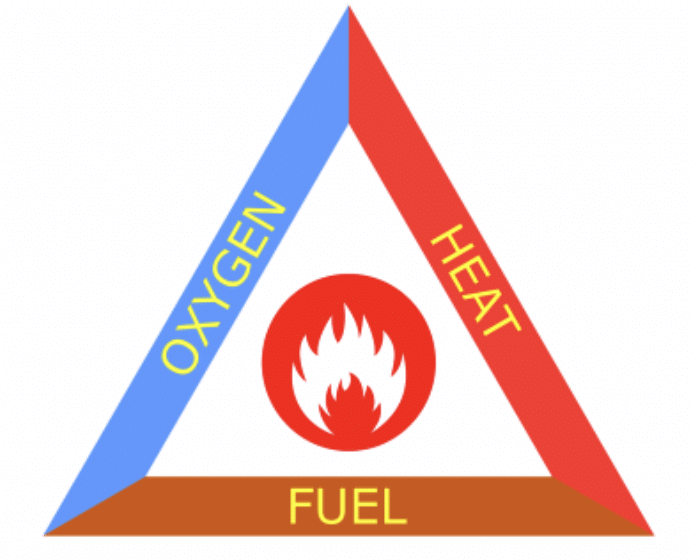
Q5. Name the three requirements of the fire triangle.
Fuel, oxygen, and heat (ignition temperature).
Q6. Which process breaks down rocks into soil over time?
Weathering.
Q7. What kind of change is melting wax?
Physical change.
Q8. What happens when magnesium ribbon is burnt in air?
When magnesium ribbon is burnt in air, it reacts with the oxygen present to form magnesium oxide. This process produces a bright flame and results in a white powder.
- The reaction can be summarised as:
- Magnesium (Mg) Oxygen (O2) → Magnesium oxide (MgO)
- Heat and light are also released during this reaction.
Q.9. What do you understand by chemical change?
A change in which one or more new substances are formed is called a chemical change.
Q.10. Why formation of manure from leaves is a chemical change?
Formation of manure from leaves is a chemical change because:
- The composition of the resulting manure is different from that of the original leaves.
- New substances are created during the decomposition process.
- This transformation involves a series of chemical reactions
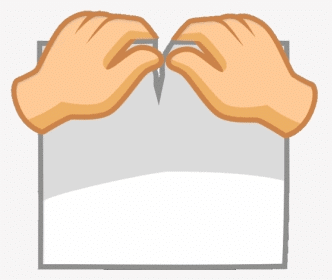
Q.11. Why tearing of paper into pieces is a physical change?
Tearing a piece of paper is a physical change because:
- Only the shape and size of the paper are altered.
- No new substance is created during this process.
Q.12. What is rusting?
If a piece of iron is left in the open for some time, it acquires a film of brownish substance. This substance is called rust the process is called rusting.
The process of rusting can be represented by the following equation: Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → rust (iron oxide Fe2O3)
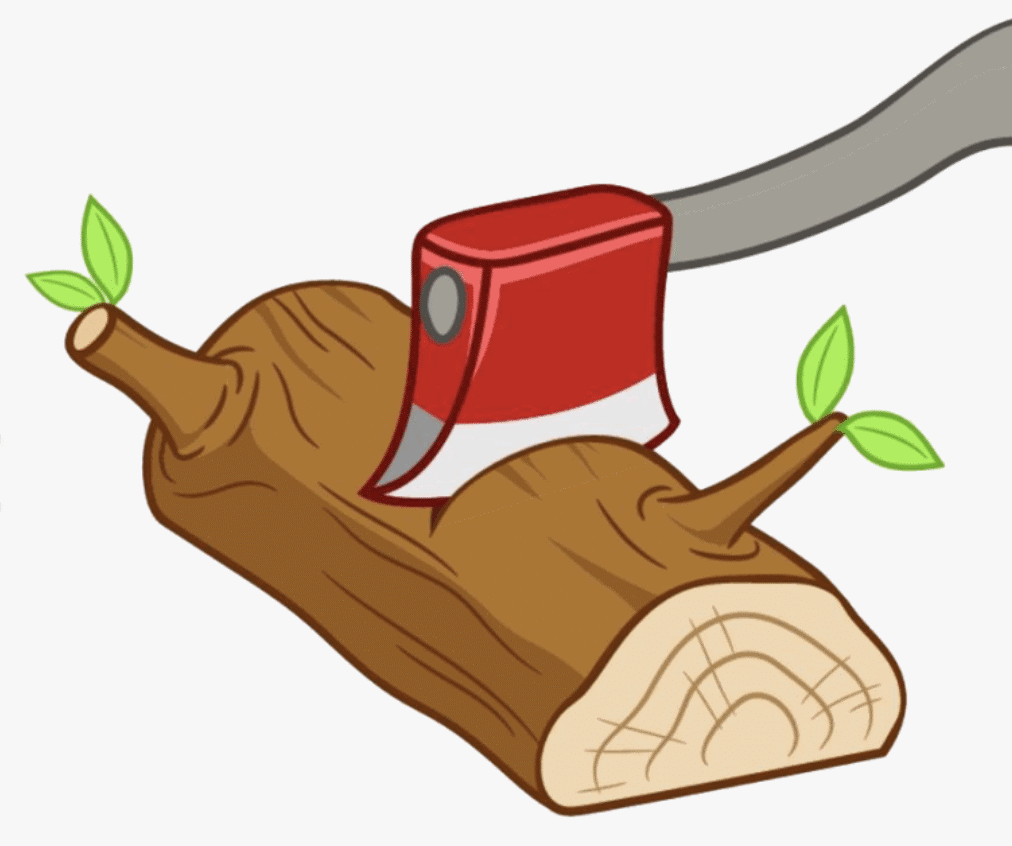
Q.13. Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Burning of wood
Burning is a non-reversible chemical change because when we burn wood new substances are formed as the carbon in the wood reacts with oxygen in the air to create ash and smoke, and energy in the form of light and heat.
Cutting of wood it into small pieces
Cutting of wood into small pieces are physical change as no new substance is formed. Only shape and size changes when wood is cut into small pieces.
Q.14. What is a physical change?
A physical change occurs when a substance alters its physical properties without forming a new substance. Key characteristics include:
- Changes can involve shape, size, colour, and state of the substance.
- These changes are generally reversible.
- No new substances are created during a physical change.
Q.15. Explain the changes that occur when a candle burns.
When a candle burns, both physical and chemical changes take place. The wax melts (physical change), evaporates, and then the vapor burns in air to form carbon dioxide and water (chemical change). Melting and re-solidifying wax are reversible, but the burning process is irreversible and forms new substances.
|
1 videos|107 docs
|
FAQs on Class 7 Science Chapter 5 Worksheet Solutions - Changes Around Us: Physical and Chemical
| 1. What are the differences between physical and chemical changes? |  |
| 2. How can we identify a chemical change? |  |
| 3. Can physical changes be reversed? |  |
| 4. What are some examples of everyday physical and chemical changes? |  |
| 5. Why is it important to understand the difference between physical and chemical changes? |  |