You won’t be able to tell the difference between a neutral and an acidic solution using turmeric, since it doesn’t show any colour change in either case.
NCERT Solutions for Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic and Neutral
Let Us Enhance Our Learning
Q1. A solution turns the red litmus paper to blue. Excess addition of which of the following solution would reverse the change?
(i) Lime water
(ii) Baking soda
(iii) Vinegar
(iv) Common salt solution
Answer: (iii) Vinegar.
Vinegar is acidic and would turn the blue litmus paper back to red, reversing the effect caused by the basic solution.
Q2. You are provided with three unknown solutions labelled A, B, and C, but you do not know which of these are acidic, basic, or neutral. Upon adding a few drops of red litmus solution to solution A, it turns blue. When a few drops of turmeric solution are added to solution B, it turns red. Finally, after adding a few drops of red rose extract to solution C, it turns green.
Based on the observations, which of the following is the correct sequence for the nature of solutions A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
Answer:
(iv) Basic, basic, and basic.
- Solution A: Red litmus turns blue, indicating a basic solution, as bases turn red litmus blue. This is correct.
- Solution B: Turmeric solution turns red. Turmeric turns red in basic solutions and remains yellow in acidic or neutral solutions. Solution B must be basic.
- Solution C: Red rose extract turns green, indicating an basic solution.Thus, the correct sequence is:
- Therefore, Solution A is basic, B is basic and C is also Basic
Q3. Observe and analyse Figs. 2.13, 2.14, and 2.15, in which red rose extract paper strips are used. Label the nature of solutions present in each of the containers.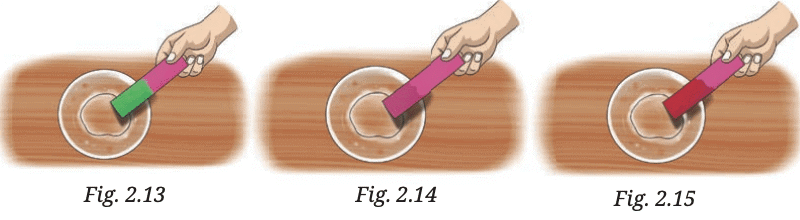
Answer: Red rose extract turns red in acidic solutions and green in basic solutions.
Fig. 2.13: The red rose extract paper strip turns green in the solution. This indicates that the solution is basic.
Fig. 2.14: The red rose extract paper strip shows no significant color change in the solution. This indicates that the solution is neutral.
Fig. 2.15: The red rose extract paper strip turns red. This indicates that the solution is acidic.
Q4. A liquid sample from the laboratory was tested using various indicators:
Based on the tests, identify the acidic or basic nature of the liquid and justify your answer.
Answer: The liquid is acidic because it turned the blue litmus paper red, which is a clear indication of an acidic solution. Additionally, no change in the turmeric paste and no change in red litmus further suggests the presence of an acid.
Blue litmus paper turns red in acidic solutions.
Turmeric paste remains unchanged in acidic solutions, as turmeric changes to red in the presence of basic solutions, but stays yellow in acidic solutions.
Therefore, these observations confirm that the liquid is acidic.
Q5. Manya is blindfolded. She is given two unknown solutions to test and determine whether they are acidic or basic. Which indicator should Manya use to test the solutions and why?
Answer: Smelling the solution after adding an olfactory indicator allows Manya to determine whether the solution is acidic or basic based on the change in smell.
Olfactory indicators are useful when visual indicators (like litmus paper) cannot be used, such as when someone is blindfolded.
Vanilla extract:
In an acidic solution, vanilla extract will have a pleasant smell.
In a basic solution, its smell may become fainter or change slightly.
Onion (used in some cases):
Onion and garlic have a strong smell that can sometimes change in the presence of bases, though they are not as commonly used as olfactory indicators.
Q6. Could you suggest various materials which can be used for writing the message on the white sheet of paper (given at the beginning of the chapter) and what could be in the spray bottle? Make a table of various possible combinations and the colour of the writing obtained.
Answer:

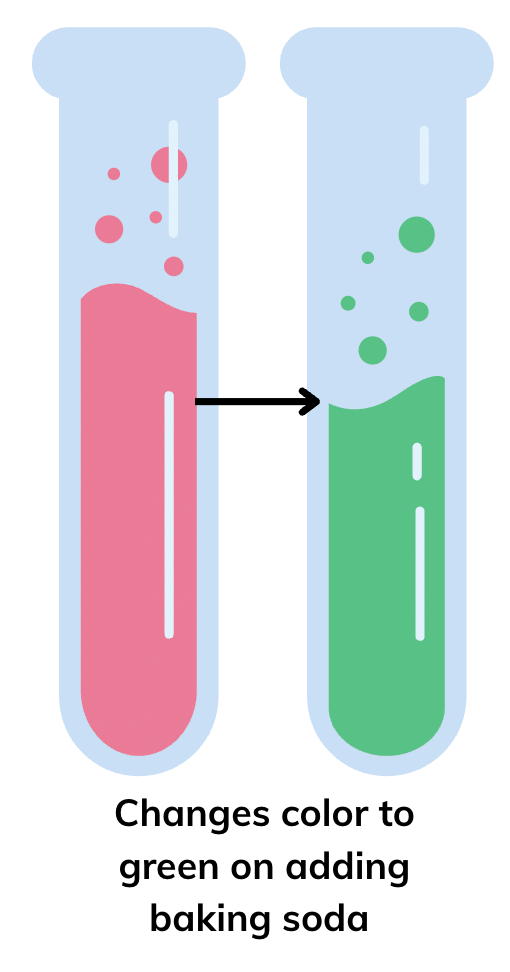
Q7. Grape juice was mixed with red rose extract; the mixture got a tint of red colour. What will happen if baking soda is added to this mixture? Justify your answer.
Answer: The colour of the solution will change from red to green, as baking soda, a basic substance, makes the mixture basic, and red rose extract turns green in basic solutions.
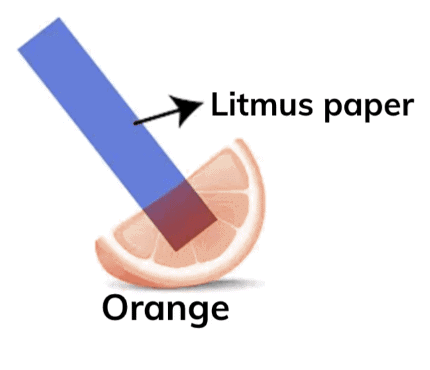
Q8. Keerthi wrote a secret message to her grandmother on her birthday using orange juice. Can you assist her grandmother in revealing the message? Which indicator would you use to make it visible?
Answer: Keerthi's grandmother can use blue litmus paper or red rose extract to detect whether the orange juice is acidic and make the message visible.
Blue Litmus Paper:
- Since orange juice is acidic, it will turn the blue litmus paper red.
- This indicates the presence of acid, and if the message was written using a substance that is invisible in neutral or basic conditions, it will become visible when the blue litmus paper turns red in the acidic environment.
Red Rose Extract:
- Red rose extract is a acid base indicator that turns red in acidic solutions.
- Since orange juice is acidic, it will cause the red rose extract to turn red, making the written message visible.

Q9. How can natural indicators be prepared? Explain by giving an example.
Answer: Natural indicators can be prepared by extracting colour pigments from natural substances like flowers, fruits, or vegetables.
For example, Preparation of Red Rose Extract
- Red rose extract is prepared by collecting fresh rose petals and washing them properly.
- The petals are then crushed and soaked in hot water.
- After some time, the mixture is filtered to get a red-colored liquid known as red rose extract.

Q10. Three liquids are given to you. One is vinegar, another is a baking soda solution, and the third is a sugar solution. Can you identify them only using turmeric paper? Explain.
Answer: Yes, you can identify the basic solution out of the three liquids (vinegar, baking soda solution, and sugar solution) using turmeric paper, as turmeric acts as a natural pH indicator. Here's how:
Vinegar (Acidic):
Turmeric paper will remain yellow when dipped in vinegar because turmeric stays yellow in acidic solutions.
So, if the turmeric paper stays yellow, the liquid is vinegar.

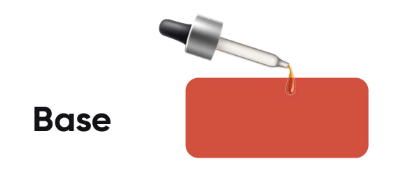
Baking Soda Solution (Basic):
Turmeric paper will turn red when dipped in a basic solution.
Since baking soda solution is basic, the turmeric paper will turn red, indicating that the liquid is a baking soda solution.
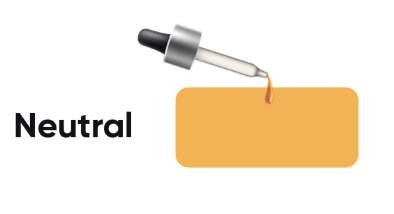
Sugar Solution (Neutral):
Turmeric paper will stay yellow in a neutral solution (like sugar solution), as it does not react to neutral substances.
If the turmeric paper remains yellow, the liquid is likely a sugar solution.
Q11. The extract of red rose turns the liquid X to green. What will the nature of liquid X be? What will happen when excess of amla juice is added to liquid X?
Answer: Liquid X is basic because the red rose extract turns green in basic solutions. Red rose extract is an indicator, and it turns red in acidic solutions and green in basic solutions.
When excess amla juice (which is acidic) is added to liquid X, the solution will become acidic. The red rose extract will then turn red in the acidic solution, indicating that the solution has now become acidic due to the addition of the amla juice.
Q12. Observe and analyse the information given in the following flowchart. Complete the missing information.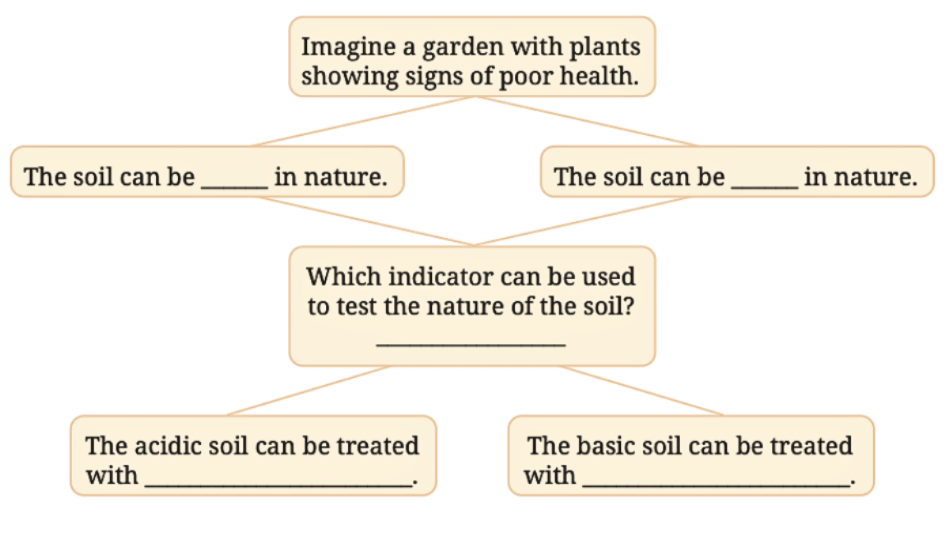
Answer: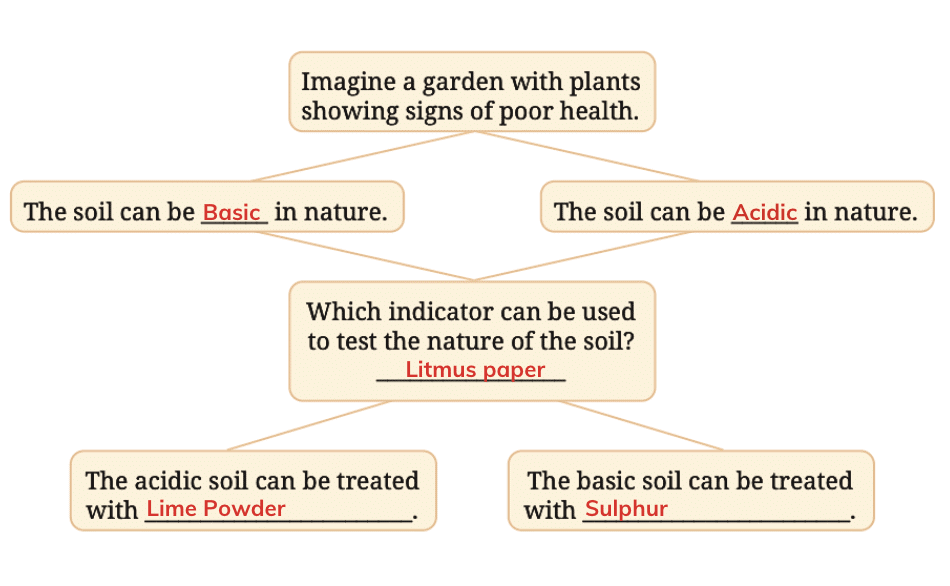
|
80 videos|322 docs|12 tests
|
FAQs on NCERT Solutions for Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic and Neutral
| 1. What are acids and bases? |  |
| 2. How can we identify acidic, basic, and neutral substances? |  |
| 3. What are some common examples of acidic and basic substances? |  |
| 4. What is the pH scale, and why is it important? |  |
| 5. Can a substance be both acidic and basic? |  |