Class 7 Science Chapter 4 Question Answers - The World of Metals and Non-metals
Short Questions and Answers
Q1: What is malleability?
Ans: Malleability is the property of materials by which they can be beaten or hammered into thin sheets. Metals like copper, aluminium, and iron possess malleability.
Q2: What happens when metals like copper and aluminium are beaten with a hammer?
Ans: Metals like copper and aluminium become flattened when beaten with a hammer, demonstrating their malleability.
Q3: What is ductility?
Ans: Ductility is the property of materials that allows them to be drawn into wires. Metals such as gold and copper are highly ductile.
Q4: What is sonority in metals?
Ans: Sonority is the property of metals to produce a ringing sound when struck. Metals like iron and steel are sonorous.
Q5: Why are metals used for cooking utensils?
Ans: Metals are good conductors of heat, which makes them ideal for cooking utensils as they quickly transfer heat to food.
Q6: What is corrosion?
Ans: Corrosion is the gradual deterioration of metals due to reactions with air, water, or other substances. For example, iron rusts when exposed to moist air.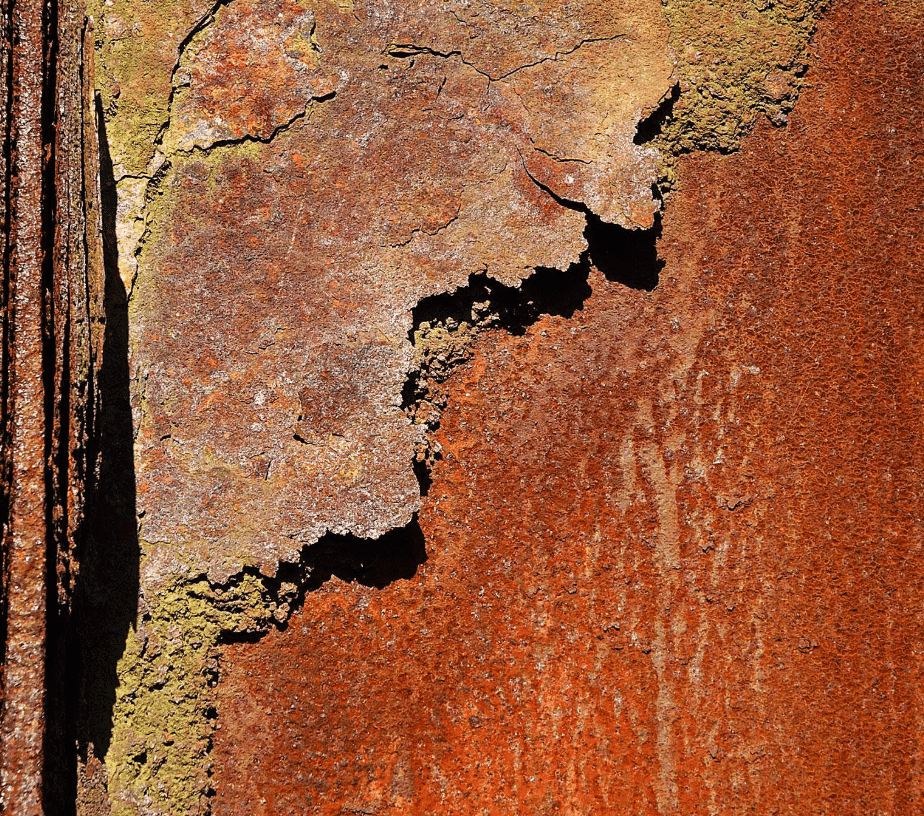
Q7: What happens when metals react with oxygen?
Ans: When metals react with oxygen, they form metal oxides, which are usually basic in nature.
Q8: Why are metals like iron and copper used in electrical wiring?
Ans: Metals like iron and copper are good conductors of electricity, making them ideal for use in electrical wiring.
Q9: Why is sodium stored in kerosene?
Ans: Sodium reacts vigorously with water and oxygen, generating a lot of heat. Storing sodium in kerosene prevents it from reacting with moisture and air.
Q10: What is the importance of metals and non-metals in everyday life?
Ans: Metals and non-metals play vital roles in daily life. Metals are used in construction, electrical devices, and tools, while non-metals like oxygen and nitrogen are essential for life and plant growth.
Long Questions and Answers:
Q1: Describe the physical properties of metals and how they differ from non-metals.
Ans: Metals generally exhibit properties like metallic lustre, malleability, ductility, sonority, and high conductivity of heat and electricity. Non-metals, on the other hand, are generally non-lustrous, brittle, and poor conductors of heat and electricity. Metals can be hammered into sheets (malleable) and drawn into wires (ductile), whereas non-metals lack these properties. Non-metals also form acidic oxides when they react with oxygen, while metals typically form basic oxides.
Q2: How does the reaction of metals and non-metals with oxygen differ?
Ans: Metals generally react with oxygen to form basic metal oxides, such as iron oxide (rust), which is alkaline in nature. Non-metals, on the other hand, form acidic oxides when they react with oxygen, such as sulfur dioxide, which forms sulfurous acid when dissolved in water. This difference in reactions is key to understanding the nature of oxides formed by metals and non-metals.
Q3: Explain the process of rusting and how it can be prevented.
Ans: Rusting is the process where iron reacts with oxygen and water to form iron oxide (rust), which causes the iron to deteriorate. Rusting can be prevented by methods such as painting, oiling, greasing, or applying a protective layer of zinc (galvanization). These methods protect the iron from direct exposure to air and water, thus preventing corrosion.
Q4: What are the applications of metals in daily life?
Ans: Metals have numerous applications in daily life due to their distinct properties. For instance, metals like copper and aluminium are used for electrical wiring due to their excellent conductivity. Iron and steel are used in construction and making tools due to their strength and malleability. Precious metals like gold and silver are used in jewelry, while aluminium is used in food packaging materials due to its malleability and low cost. Metals are also used in the manufacturing of machinery, vehicles, and electronics.
Q5: Discuss the uses of non-metals in everyday life.
Ans: Non-metals like oxygen are essential for respiration, making them crucial for life. Nitrogen is used in fertilizers to enhance plant growth. Carbon is an important element in organic molecules and fuels. Chlorine is used in water purification, while iodine is used as an antiseptic for wounds. Non-metals are also found in medicines, food preservation, and industrial applications like rubber manufacturing and water treatment.
|
80 videos|319 docs|12 tests
|
FAQs on Class 7 Science Chapter 4 Question Answers - The World of Metals and Non-metals
| 1. What are the main differences between metals and non-metals? |  |
| 2. Why are metals considered good conductors of electricity? |  |
| 3. What are some common uses of non-metals in everyday life? |  |
| 4. How do metals and non-metals react with acids? |  |
| 5. What is the significance of the reactivity series in understanding metals and non-metals? |  |

















