Heat and Temperature Chapter Notes | Technical Science for Grade 10 PDF Download
| Table of contents |

|
| Temperature |

|
| Heat is Energy in Transfer |

|
| Applications of Heat Transfer |

|
| Heat Energy |

|
| Points to Remember |

|
| Difficult Words |

|
| Summary |

|
Temperature
This section introduces temperature, its measurement, the Celsius and Kelvin scales, and various types of thermometers used to measure it.
Definition of Temperature
Concept: Temperature measures how hot or cold a substance is, reflecting the average kinetic energy of its particles.Explanation:
- In fluids (liquids or gases), temperature depends on the kinetic energy of moving particles.
- In solids, it depends on the kinetic energy of vibrating particles.
- Higher kinetic energy means a hotter substance; lower kinetic energy means a colder substance.
Example: Hot steel (reddish or yellow/white) has particles with higher kinetic energy than cooler steel; water at 100°C has particles with more kinetic energy than water at 0°C.
Measuring Temperature
Instrument: Temperature is measured using a thermometer.Unit: Degrees Celsius (°C) is the standard unit in most countries; the SI unit is kelvin (K), used mainly by scientists.
Celsius Scale: Developed by Anders Celsius over 250 years ago, dividing the range between water’s freezing point (0°C) and boiling point (100°C) into 100 equal parts.
Key temperatures:
- Normal body temperature: ~37°C.
- Comfortable air temperature: 20°C (“twenty is nice”); 30°C is hot, 10°C is cool, 0°C is ice.
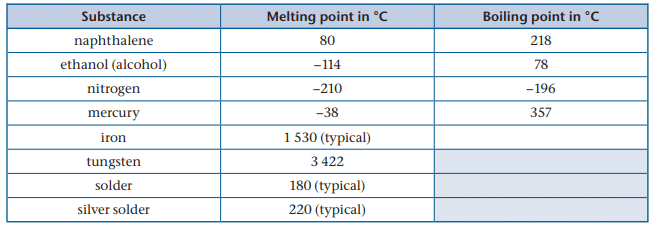
Melting and Boiling Points:
Some melting and boiling points in degrees Celsius:
Types of Thermometers
Overview: Four main types are used: bulb thermometers, thermoelectric thermometers, bimetallic thermometers, and temperature strips.
Bulb Thermometers:
Structure: Glass tube with a liquid-filled bulb at one end, connected to a thin, sealed tube; liquid moves up or down as temperature changes.
Liquids:
- Mercury (silver-colored, used in clinics but not schools).
- Ethanol/alcohol (colored red or blue, used in labs).
- Water (early thermometers, limited to above 0°C).
Principle: Liquid volume changes with temperature (expands when heated, contracts when cooled), moving along a calibrated scale.
Calibration: Ensures accurate temperature readings; requires practice to read correctly.
Thermoelectric Thermometers:
Use: Electronic devices in appliances and industry, replacing bulb thermometers due to speed, cost, and readability.
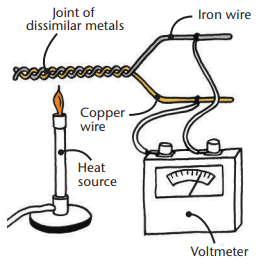 How a thermocouple worksSensors:
How a thermocouple worksSensors:
- Thermocouple: Two different conductors joined at one end; temperature difference creates a voltage, converted to a temperature reading.
- Thermistor: Resistance changes with temperature; voltage changes are converted to temperature, shown on a digital screen.
- Accuracy: Thermistors are generally more accurate than thermocouples.
Examples: Digital thermometers, gas cooker sensors.
Bimetallic Thermometers:
Structure: Two metal strips with different expansion rates joined together; heating causes bending due to unequal expansion.
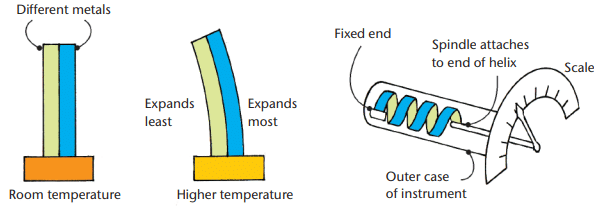 Bimetallic strip and bimetallic thermometerUses:
Bimetallic strip and bimetallic thermometerUses:
- Moves a pointer on a dial thermometer.
- Acts as a thermostat, switching circuits on/off (e.g., in heaters when too hot/cold).
Characteristics: Tough and reliable but less accurate.
Temperature Strips:
Structure: Flexible plastic with liquid crystals, used for surface temperatures in labs, hospitals, and homes.
 A strip thermometerPrinciple:
A strip thermometerPrinciple:
- Light waves reflect off crystals, with interference depending on crystal spacing.
- Temperature changes alter crystal spacing, changing the reflected light’s color.
Example: Stick-on strips showing temperature via color changes.
Kelvin Temperature Scale
Overview: SI unit for temperature, used in scientific contexts like thermodynamics; extends the Celsius scale to absolute zero (-273°C).Notation: Written as K (e.g., 273 K), not “degrees Kelvin.”
Relation to Celsius:
- One kelvin equals one degree Celsius in size.
- Key points: Ice melts at 273 K, water boils at 373 K.
Conversion Formulas:
- Celsius to Kelvin: T = (t + 273) K
Example: 100°C = 100 + 273 = 373 K. - Kelvin to Celsius: t = (T - 273) °C
Example: 100 K = 100 - 273 = -173°C.
Heat is Energy in Transfer
This section explains heat as energy transferred between objects and the three methods of heat transfer: conduction, convection, and radiation.
Definition of Heat
- Concept: Heat is energy in transfer, moving from an object at a higher temperature to one at a lower temperature.
- Example: Sitting near a fire warms you as heat energy transfers from the fire to your body.
Methods of Heat Transfer
Overview: Heat energy transfers in three ways: conduction, convection, and radiation.
Conduction:
- Definition: Heat transfer in solids through particle collisions.
Process:
- A rapidly vibrating particle transfers kinetic energy to a slower-vibrating neighbor.
- Collisions cause neighboring particles to vibrate faster, spreading energy through the solid.
Example: A pot’s handle warms when the pot is heated, as energy transfers from the base to the handle.
Convection:
Definition: Heat transfer in fluids (liquids or gases) through the motion of hotter, less dense fluid.
Process:
- Heating a fluid reduces its density, causing hotter parts to rise through colder, denser parts.
- This creates a convection current, transferring energy.
Example: In a pot of water, heated water rises, and cooler water sinks, shown by purple patterns from dissolved potassium permanganate.
Radiation (Thermal Radiation):
Definition: Heat transfer via electromagnetic waves, requiring no contact between objects.
Process:
- All objects emit radiation; hotter objects emit more.
- Energy transfers through a vacuum or gas without particle interaction.
Example: Feeling heat around a pot or seeing hotter parts as orange in a thermal imaging camera.
Applications of Heat Transfer
Examples:- Hot Water Pipes: Heat from pipes warms surrounding air via conduction (through steel), convection (air movement), and radiation.
- Cooking: Gas flame heats a pot’s metal base (conduction), and water circulates heat (convection) to cook food evenly.
- Car Engine: Petrol combustion heats the engine block (conduction), and water in the radiator circulates to cool it (convection).
Significance: Understanding heat transfer helps design systems like heating, cooking, and cooling mechanisms.
Heat Energy
This section focuses on measuring heat energy, its unit (joules), and calculations for specific scenarios.

Measuring Heat Energy
- Unit: Heat, a form of energy, is measured in joules (J).
- Context: Quantifying heat energy is crucial for tasks like cooking or fueling vehicles (e.g., wood for a fire, petrol for a car trip).
Calculating Heat Energy
Key Fact: It takes 4.184 J to raise the temperature of 1 ml of water by 1°C.Example Calculation: Heating 250 ml of water from 20°C to 50°C for coffee:
- Temperature change: 50 - 20 = 30°C.
- Energy for 1 ml by 1°C: 4.184 J.
- Energy for 250 ml by 1°C: 4.184 × 250 = 1046 J.
- Energy for 250 ml by 30°C: 1046 × 30 = 31,380 J ≈ 30,000 J (to nearest 10,000 J).
Additional Fact: Melting 1 g of ice to water (without temperature change) requires 334 J, indicating significant energy for phase changes.
Points to Remember
- Temperature measures a substance’s hotness, reflecting the average kinetic energy of its particles (higher in hotter substances).
- Thermometers measure temperature in degrees Celsius (°C); the SI unit is kelvin (K).
- The Celsius scale sets water’s freezing point at 0°C and boiling point at 100°C; normal body temperature is ~37°C.
- Bulb thermometers use liquids (ethanol, mercury) that expand/contract with temperature; thermoelectric thermometers (thermocouples, thermistors) are faster and digital.
- Bimetallic thermometers use metal strips that bend with heat; temperature strips show color changes via liquid crystals.
- Kelvin scale extends Celsius to -273°C (0 K); convert using T = (t + 273) K or t = (T - 273) °C.
- Heat is energy transferred from higher to lower temperature objects via conduction, convection, or radiation.
- Conduction transfers heat in solids through particle collisions; convection occurs in fluids via rising hot, less dense parts.
- Radiation transfers heat via electromagnetic waves, requiring no contact, effective in vacuums or gases.
- Heat energy is measured in joules (J); 4.184 J raises 1 ml of water by 1°C; 334 J melts 1 g of ice.
- Applications include heating homes (conduction, convection, radiation), cooking (convection), and cooling car engines (conduction, convection).
Difficult Words
- Temperature: A measure of how hot or cold a substance is, based on the average kinetic energy of its particles.
- Thermometer: A device (e.g., bulb, thermoelectric) used to measure temperature.
- Celsius Scale: A temperature scale where water freezes at 0°C and boils at 100°C.
- Kelvin Scale: The SI temperature scale starting at absolute zero (-273°C), used in science; 1 K = 1°C in size.
- Bulb Thermometer: A glass thermometer with a liquid (e.g., ethanol) that expands/contracts with temperature.
- Thermoelectric Thermometer: An electronic device using thermocouples or thermistors to measure temperature digitally.
- Bimetallic Thermometer: A device with two metals that bend when heated, used in thermometers or thermostats.
- Temperature Strip: A plastic strip with liquid crystals that change color with temperature.
- Heat: Energy transferred from a hotter to a colder object.
- Conduction: Heat transfer in solids via particle collisions.
- Convection: Heat transfer in fluids through the movement of hotter, less dense parts.
- Radiation: Heat transfer via electromagnetic waves, requiring no contact.
- Joule (J): The unit of heat energy.
- Thermodynamics: The science of heat energy, its transfer, and its relation to mechanical work.
Summary
Chapter 16 explores heat and temperature, fundamental to thermodynamics. Temperature, a measure of a substance’s hotness, reflects the average kinetic energy of its particles, measured in degrees Celsius (°C) or kelvin (K). Thermometers (bulb, thermoelectric, bimetallic, temperature strips) measure temperature, with Celsius based on water’s freezing (0°C) and boiling (100°C) points, and Kelvin extending to absolute zero (-273°C). Heat, energy in transfer, moves from hotter to colder objects via conduction (in solids through particle collisions), convection (in fluids via rising hot parts), or radiation (via electromagnetic waves, no contact needed). Heat energy, measured in joules (J), requires 4.184 J to raise 1 ml of water by 1°C and 334 J to melt 1 g of ice. These principles apply to heating systems, cooking, and engine cooling, laying the foundation for studying heat engines in Grade 11.
|
1 videos|77 docs|5 tests
|
FAQs on Heat and Temperature Chapter Notes - Technical Science for Grade 10
| 1. What is the difference between heat and temperature? |  |
| 2. What are the main methods of heat transfer? |  |
| 3. How is heat energy utilized in everyday applications? |  |
| 4. What are some key points to remember about heat and temperature in Grade 10? |  |
| 5. What are some difficult words related to heat and temperature that students should know? |  |


















