Q1: Which metal is commonly used to make food packaging materials as it is cheaper, and its thin sheets can be folded easily into any shape?
(i) Aluminium
(ii) Copper
(iii) Iron
(iv) Gold
Ans: (i) Aluminium
Q2: Which of the following metal catches fire when it comes in contact with water?
(i) Copper
(ii) Aluminium
(iii) Zinc
(iv) Sodium
Ans: (iv) Sodium
Q3: State with reason(s) whether the following statements are True [T] or False [F].
(i) Aluminium and copper are examples of non-metals used for making utensils and statues.
Ans: False
(ii) Metals form oxides when combined with oxygen, the solution of which turns blue litmus paper to red.
Ans: False
(iii) Oxygen is a non-metal essential for respiration.
Ans: True
(iv) Copper vessels are used for boiling water because they are good conductors of electricity.
Ans: False
Q4: Why are only a few metals suitable for making jewellery?
Ans: Only a few metals like gold, silver, and platinum are suitable for making jewellery because they have special properties:
Malleability: These metals can be easily shaped into fine designs without breaking.
Ductility: They can be drawn into thin wires, which is important for making delicate jewellery pieces.
Lustrous: These metals have a shiny and attractive appearance, which makes them look beautiful in jewellery.
Resistant to corrosion: They do not rust or tarnish easily, so the jewellery remains durable and keeps its shine over time.
These properties make gold, silver, and platinum perfect choices for creating attractive and long-lasting jewellery.
Q5: Match the uses of metals and non-metals given in Column I with the jumbled names of metals and non-metals given in Column II.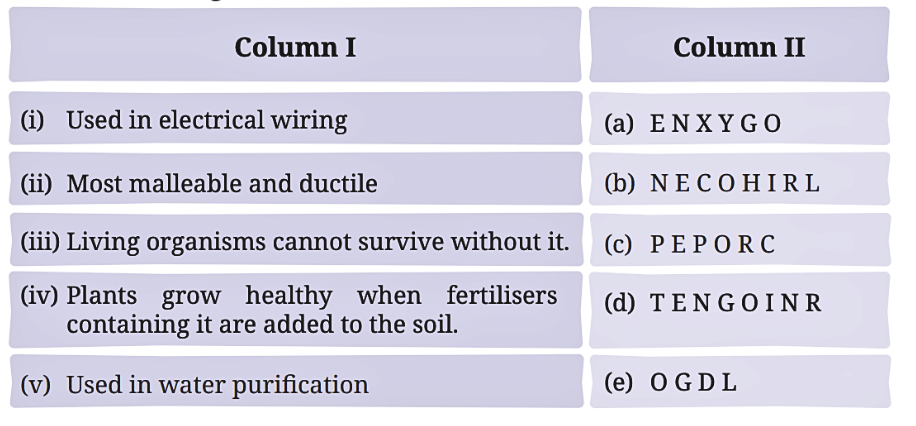
Ans: 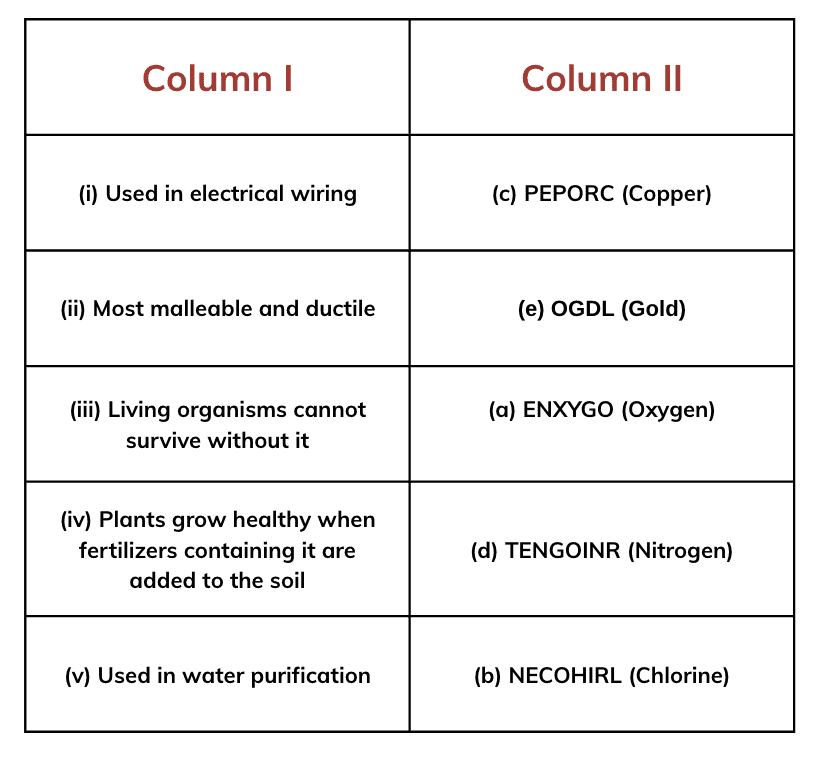
Q6: What happens when oxygen reacts with magnesium and sulfur? What are the main differences in the nature of products formed?
Ans: 1. Oxygen Reacting with Magnesium
- When oxygen reacts with magnesium, it forms magnesium oxide, which is a basic oxide.
- Magnesium + Oxygen → Magnesium Oxide
- Basic oxides are generally properties of metal oxides.
2. Oxygen Reacting with Sulfur:
- When oxygen reacts with sulfur, it produces sulfur dioxide, which is an acidic oxide.
- Sulfur + Oxygen → Sulfur Dioxide
- Acidic oxides are usually associated with non-metal oxides.
The main distinction is that magnesium oxide is basic, while sulfur dioxide is acidic in nature.
Q7: Complete the following flow chart: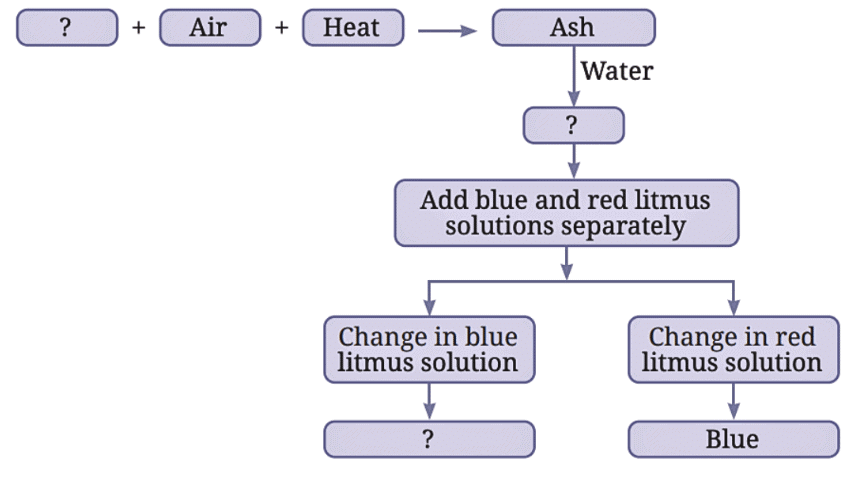 Ans:
Ans: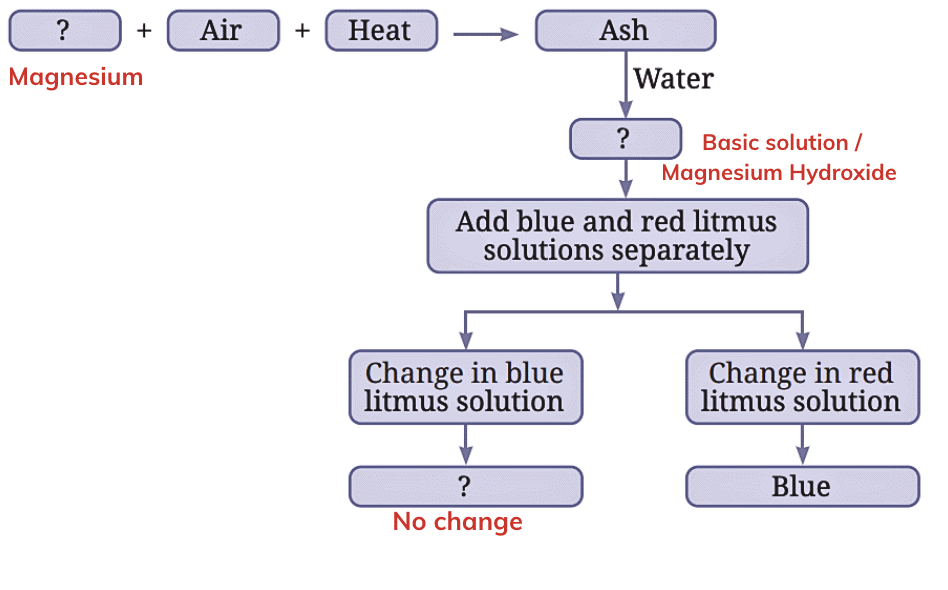
Q8: You are provided with the following materials. Discuss which material would be your choice to make a pan that is most suitable for boiling water and why?
Ans: Copper is an excellent conductor of heat, ensuring quick and even heating, which is essential for efficiently boiling water. It is also durable and resistant to corrosion, making it ideal for long-term use in cooking applications where the pan is exposed to high temperatures and water. Iron, while a good conductor, is prone to rusting, requiring maintenance to prevent corrosion, which makes it less suitable. Sulfur, coal, plastic, wood, and cardboard are poor conductors of heat and cannot withstand the high temperatures needed for boiling water, with some being brittle or flammable.
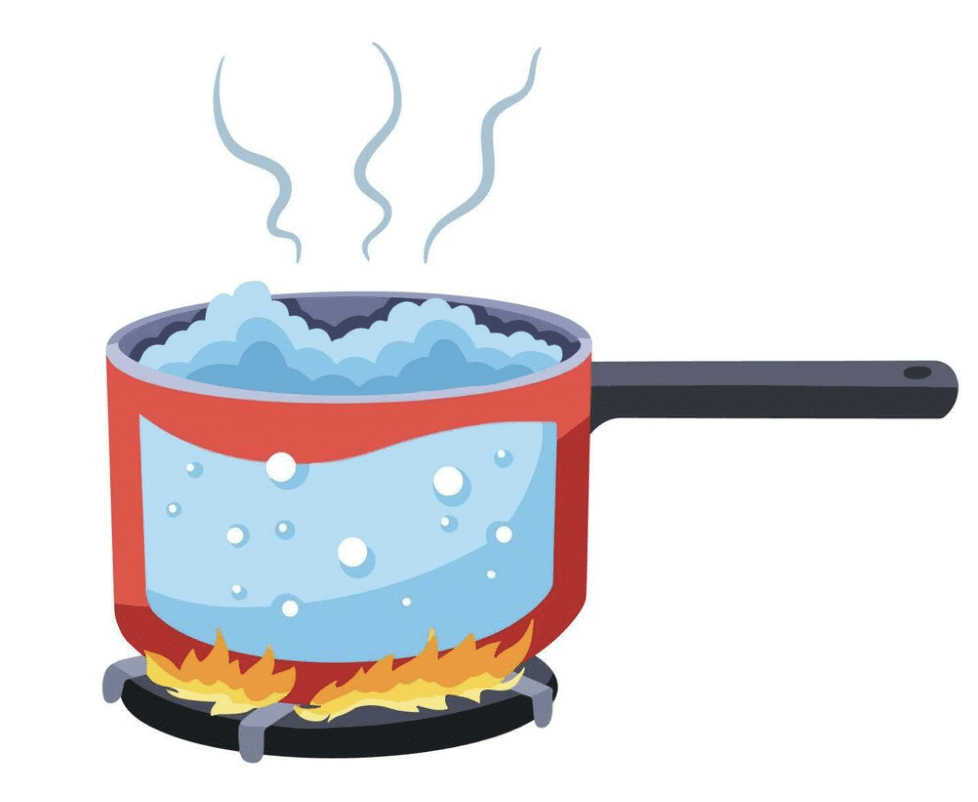
Copper is the optimal material for making a pan suitable for boiling water
Q9: You are provided with three iron nails, each dipped in oil, water, and vinegar. Which iron nail will not rust, and why?
Ans: Rusting of iron occurs when it reacts with water and oxygen from the air, forming iron oxide (rust).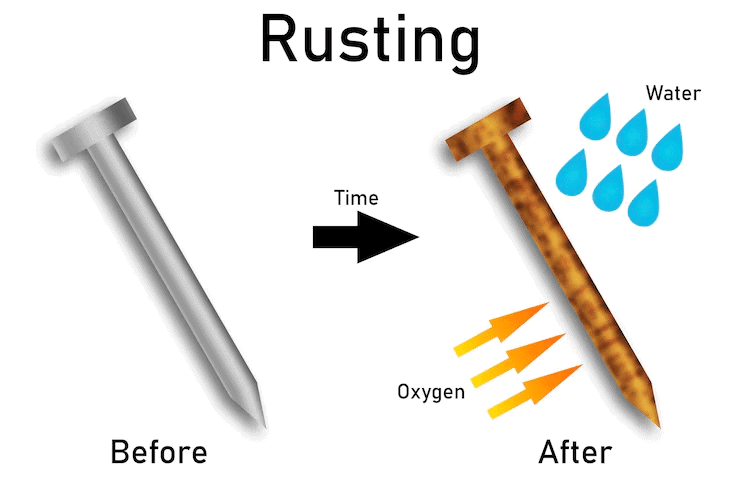
Rusting occurs when iron reacts with water and oxygen, forming iron oxide (rust). The oil on the iron nail creates a protective barrier, preventing moisture and oxygen from reaching the iron surface, thus inhibiting rusting. The nail in water will rust because water provides the moisture needed for the reaction. The nail in vinegar will rust faster due to the acetic acid, which accelerates corrosion by enhancing the reaction between iron, water, and oxygen. The iron nail dipped in oil will not rust.
Q10: How do the different properties of metals and non-metals determine their uses in everyday life?
Ans: Properties of metals and non-metals directly determine their practical uses in our everyday lives.
Metals
Metals can be hammered into thin sheets without breaking. This property makes metals ideal for making tools and utensils, as they can be shaped into different forms easily.
Metals can be drawn into wires. This is why metals are used to make wires for electrical circuits.
Metals produce a ringing sound when struck, which is why they are used in musical instruments and bells.
Metals like copper and aluminum are excellent conductors of heat and electricity. This makes them ideal for use in wires, cooking utensils, and heating systems.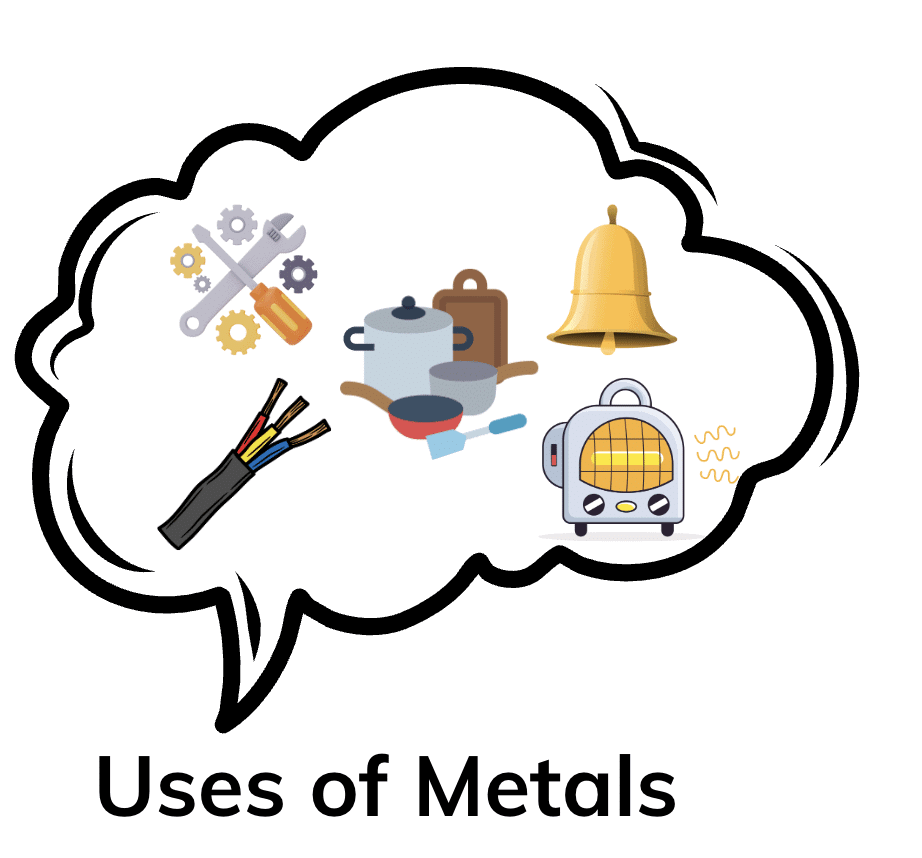
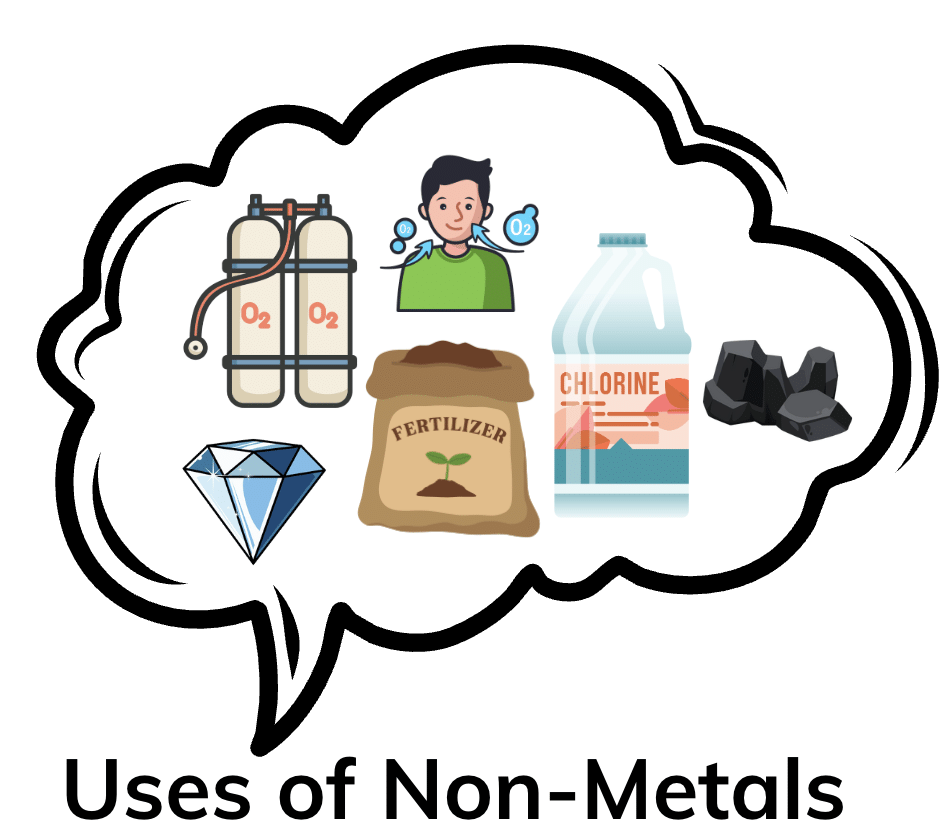
Non-Metals
- Non-metals are generally poor conductors of heat and electricity, which is why they are used in insulation materials (e.g., rubber for insulating wires).
- Non-metals like oxygen are highly reactive with substances like fuel, making them essential in respiration (oxygen is used by humans and animals to release energy).
- Nitrogen is inert and is used to make fertilisers (reacts with hydrogen to form ammonia), while sulfur is used in making disinfectants and medicines due to its chemical reactivity.
- Many non-metals, like oxygen and nitrogen, are gases at room temperature, which makes them essential in various life processes, such as breathing and fertilisation.
Q11: One of the methods of protecting iron from getting rusted is to put a thin coating of zinc metal over it. Since sulfur does not react with water, can it be used for this purpose? Justify your answer.
Ans: No, sulfur cannot be used to protect iron from rusting because:
Zinc is used for this purpose because it forms a protective coating over the iron. This coating reacts with oxygen in the air to form a layer of zinc oxide, which prevents water and oxygen from reaching the iron and causing rusting.
Sulfur, on the other hand, does not react with water and air in the same way. It does not form a protective barrier around the iron. Since sulfur does not create a coating that blocks moisture and oxygen, it cannot prevent rusting like zinc can.
Q12: An ironsmith heats iron before making tools. Why is heating necessary in this process?
Ans: Heating is necessary because it makes the iron more malleable (easier to bend and shape). When the iron is heated, it becomes softer and more flexible, which allows the ironsmith to shape it into tools, such as axes, tongs, and other items.
This process is much easier when the metal is red hot because at this temperature, the iron is softer and can be shaped with less force. Therefore, heating helps the ironsmith shape the iron into the desired tools efficiently.





 Ans:
Ans:























