NCERT Based Activity: Matter in Our Surroundings | Science Class 9 PDF Download
Activity 1.1: Dissolving Salt or Sugar in Water
Description:
- Take a 100 mL beaker.
- Fill half the beaker with water and mark the level of water.
- Dissolve some salt/sugar with the help of a glass rod.
- Observe any change in water level.
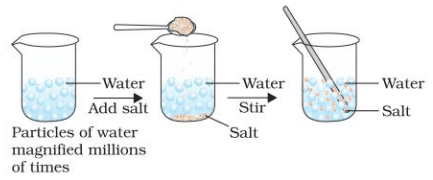 When we dissolve salt in water, the particles ofsalt get into the spaces between particles of water.
When we dissolve salt in water, the particles ofsalt get into the spaces between particles of water. - What do you think has happened to the salt? Where does it disappear? Does the level of water change?
Ans: Observation: The water level does not change significantly after dissolving salt or sugar.
Explanation: The salt or sugar particles dissolve in water by occupying the spaces between water particles. The salt/sugar does not disappear; it spreads evenly throughout the water, forming a solution. This demonstrates that matter is made up of particles, and there is space between the particles of water that allows the salt/sugar particles to fit in without increasing the volume significantly.
Activity 1.2: Dilution of Potassium Permanganate to Show Particle Size
- Take 2-3 crystals of potassium permanganate and dissolve them in 100 mL of water.
- Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
- Repeat the dilution process 5 to 8 times.
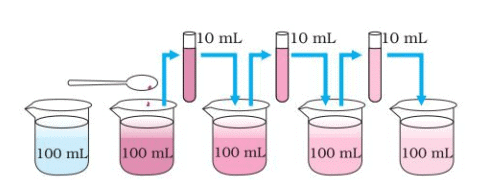 Estimating how small are the particles of matter. With every dilution, though the colour becomes light, it is still visible.
Estimating how small are the particles of matter. With every dilution, though the colour becomes light, it is still visible. - Is the water still coloured?
Ans: Observation: Even after multiple dilutions (5 to 8 times), the water remains slightly coloured, though the colour becomes lighter with each dilution.
Explanation: A few crystals of potassium permanganate can colour a large volume of water because they consist of millions of tiny particles that divide and spread during dilution. This shows that particles of matter are extremely small, beyond our imagination, as even after repeated dilution, the colour persists.
Activity 1.3: Diffusion of Aroma from Incense Stick
Description:
- Put an unlit incense stick in a corner of your class. How close do you have to go near it to get its smell?
- Now light the incense stick. What happens? Do you get the smell sitting at a distance?
- Record your observations.

Ans: Observation:
- With an unlit incense stick, you need to be very close to detect its smell.
- After lighting the incense stick, the smell can be detected from a distance, even across the room.
Explanation: The particles of the incense stick’s aroma are released more readily when heated (lit), allowing them to move faster and diffuse through the air. This demonstrates that particles of matter are continuously moving and that their movement increases with heat, enabling the smell to spread over a larger distance.
Activity 1.4: Diffusion of Ink and Honey in Water
Description:
- Take two glasses/beakers filled with water.
- Put a drop of blue or red ink slowly and carefully along the sides of the first beaker and honey in the same way in the second beaker.
- Leave them undisturbed.
- What do you observe immediately after adding the ink drop?
- What do you observe immediately after adding a drop of honey?
- How many hours or days does it take for the colour of ink to spread evenly throughout the water?

Ans: Observation:
- Immediately after adding the ink, it starts to spread slowly, forming streaks or clouds in the water.
- Immediately after adding honey, it sinks to the bottom and spreads very slowly, if at all, due to its higher viscosity.
- The ink colour spreads evenly throughout the water within a few hours, depending on the water’s temperature and lack of disturbance.
Explanation: Ink particles diffuse into water because they are smaller and less viscous, allowing them to move into the spaces between water particles. Honey, being viscous, diffuses much more slowly. This activity illustrates that particles of matter are in constant motion and can intermix through diffusion, with the rate depending on the substance’s properties.
Activity 1.5: Effect of Temperature on the Rate of Diffusion
Description:
- Drop a crystal of copper sulphate or potassium permanganate into a glass of hot water and another containing cold water. Do not stir the solution. Allow the crystals to settle at the bottom.
- What do you observe just above the solid crystal in the glass?
- What happens as time passes?
- What does this suggest about the particles of solid and liquid?
- Does the rate of mixing change with temperature? Why and how?
Ans: Observation:
- Just above the crystal, a coloured layer forms as the crystal begins to dissolve, with the colour spreading upward.
- Over time, the colour spreads throughout the water, faster in the hot water than in the cold water.
- The hot water shows a faster and more uniform spread of colour compared to the cold water.
Explanation: The particles of the crystal dissolve and diffuse into the water, moving into the spaces between water particles. In hot water, the particles have higher kinetic energy, leading to faster movement and diffusion. This suggests that particles of matter are in constant motion, and their kinetic energy (and thus diffusion rate) increases with temperature. The faster mixing in hot water is due to the increased kinetic energy of particles at higher temperatures.
Activity 1.6: Demonstration of Forces of Attraction Using Human Chains
Description:
- Play a game in the field with four groups forming human chains:
- First group: Hold each other from the back and lock arms like Idu-Mishmi dancers.
- Second group: Hold hands to form a human chain.
- Third group: Form a chain by touching each other with only fingertips.
- Fourth group: Try to break the three human chains into smaller groups.

- Which group was the easiest to break? Why? In which group did the particles hold each other with the maximum force?
Ans: Observation:
- The third group (fingertip chain) is the easiest to break.
- The first group (locked arms) is the hardest to break.
Explanation: The strength of the human chain represents the force of attraction between particles. The fingertip chain has the weakest attraction (like gases), making it easy to break. The locked-arm chain has the strongest attraction (like solids), making it the hardest to break. The hand-holding chain (like liquids) has an intermediate force. This illustrates that particles of matter attract each other, with the strength of attraction being highest in solids, intermediate in liquids, and lowest in gases.
Activity 1.7: Comparing Force of Attraction in Solids
Description:
- Take an iron nail, a piece of chalk, and a rubber band.
- Try breaking them by hammering, cutting, or stretching.
- In which of the above three substances do you think the particles are held together with greater force?
Ans: Observation:
- The iron nail is very difficult to break, requiring significant force.
- The chalk breaks easily when hammered or cut.
- The rubber band stretches and may break with enough force but is less rigid than the nail or chalk.
Explanation: The iron nail has the strongest forces of attraction between its particles, making it very hard to break (representing a typical solid with strong intermolecular forces). Chalk has weaker forces, allowing it to break more easily. The rubber band, while flexible, has moderate forces that allow stretching but resist breaking to some extent. Thus, the iron nail’s particles are held together with the greatest force.
Activity 1.8: Surface Cohesion in Water
Description:
- Take some water in a container, try cutting the surface of water with your fingers.
- Were you able to cut the surface of water? What could be the reason behind the surface of water remaining together?

Ans: Observation: You cannot cut the surface of water; it flows back together immediately.
- Explanation: The particles of water are attracted to each other due to cohesive forces (intermolecular attractions). These forces cause the water molecules to stick together, preventing the surface from being permanently cut. This demonstrates that particles of matter, even in the liquid state, have forces of attraction that keep them together.
Activity 1.9: Properties of Solids – Shape, Volume, and Compressibility
Description:
- Collect a pen, a book, a needle, and a piece of wooden stick.
- Sketch the shape of the articles by moving a pencil around them.
- Do all these have a definite shape, distinct boundaries, and a fixed volume?
- What happens if they are hammered, pulled, or dropped?
- Are these capable of diffusing into each other?
- Try compressing them by applying force. Are you able to compress them?
Ans: Observation:
- All items (pen, book, needle, wooden stick) have a definite shape, distinct boundaries, and fixed volume.
- When hammered, pulled, or dropped, they may break or dent but do not change shape easily.
- They do not diffuse into each other.
- They cannot be compressed significantly by applying force.
Explanation: These are solids, which have a definite shape, fixed volume, and distinct boundaries due to strong forces of attraction between particles. Solids are rigid, resist compression, and do not diffuse into each other because their particles are tightly packed and have minimal space between them.
Activity 1.10: Properties of Liquids – Shape, Volume, and Flow
Description:
- Collect water, cooking oil, milk, juice, a cold drink, and containers of different shapes. Put a 50 mL mark on the containers.
- What will happen if these liquids are spilt on the floor?
- Measure 50 mL of any one liquid and transfer it into different containers. Does the volume remain the same? Does the shape of the liquid remain the same?
- When you pour the liquid from one container to another, does it flow easily?
Ans: Observation:
- If spilt, the liquids spread out on the floor, taking the shape of the surface.
- The volume of 50 mL remains the same when transferred to different containers.
- The shape of the liquid changes to match the container’s shape.
- The liquids flow easily when poured.
Explanation: Liquids have a fixed volume but no fixed shape, taking the shape of the container they are in. They flow easily due to weaker forces of attraction between particles compared to solids, allowing particles to slide past each other. This demonstrates the fluid nature of liquids.
Activity 1.11: Compressibility of Solids, Liquids, and Gases
Description:
- Take three 100 mL syringes, close their nozzles with rubber corks.
- Remove pistons, leave one syringe empty, fill the second with water, and the third with chalk pieces.
- Insert pistons back and try to compress the content by pushing the piston.
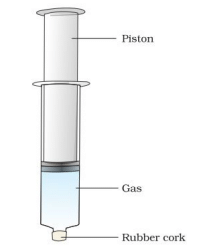
- What do you observe? In which case was the piston easily pushed in? What do you infer?
Ans: Observation:
- The piston in the empty syringe (containing air) is easily pushed in.
- The piston in the water-filled syringe is very hard to push.
- The piston in the chalk-filled syringe cannot be pushed significantly.
Explanation: Air (a gas) is highly compressible because its particles are far apart with large spaces between them. Water (a liquid) is nearly incompressible due to closely packed particles. Chalk (a solid) is not compressible because its particles are tightly bound with minimal space. This shows that gases are highly compressible compared to liquids and solids.
Activity 1.12: Change of State – Melting and Boiling Points of Water
Description:
- Take about 150 g of ice in a beaker and suspend a thermometer in contact with the ice.
- Heat the beaker on a low flame.
- Note the temperature when ice starts melting and when all ice has converted to water.
- Heat while stirring until the water boils, noting the temperature when most water has vaporized.
- Record observations for solid-to-liquid and liquid-to-gas conversions.
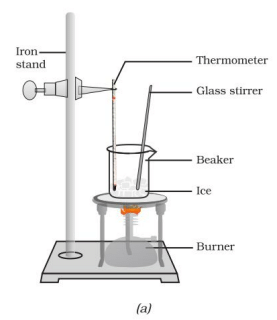
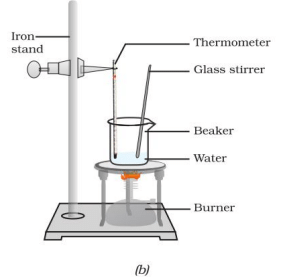 Fig. 1.6: (a) Conversion of ice to water, (b) conversion of water to water vapour
Fig. 1.6: (a) Conversion of ice to water, (b) conversion of water to water vapour
Ans: Observation:
- The ice starts melting at 0°C (273 K), and the temperature remains constant at 0°C until all ice melts.
- After all ice melts, the temperature of water rises until it reaches 100°C (373 K), where it boils and remains constant until most water vaporizes.
Explanation: At the melting point (0°C), the heat energy (latent heat of fusion) is used to overcome the forces of attraction between ice particles, changing the state from solid to liquid without a temperature increase. At the boiling point (100°C), the heat energy (latent heat of vaporization) is used to break the forces holding water molecules together, converting liquid to gas. This illustrates that temperature remains constant during state changes due to latent heat absorption.
Activity 1.13: Sublimation of Camphor
Description:
- Take some camphor, crush it, and put it in a china dish.
- Put an inverted funnel over the dish with a cotton plug on the stem.
- Heat slowly and observe.
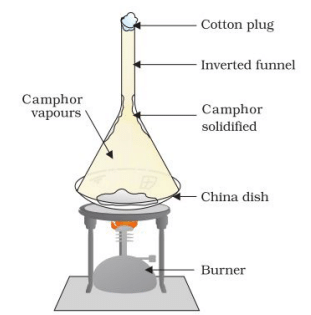 Sublimation of camphor
Sublimation of camphor - What do you infer?
Ans: Observation: The camphor disappears from the dish, and some may deposit as a solid on the inner surface of the funnel.
Explanation: Camphor undergoes sublimation, changing directly from a solid to a gas without becoming a liquid. The gas then cools and deposits back as a solid on the funnel’s surface (deposition). This demonstrates that some substances can change directly from solid to gas or gas to solid, bypassing the liquid state.
Activity 1.14: Factors Affecting the Rate of Evaporation
Description:
- Take 5 mL of water in a test tube and keep it near a window or under a fan.
- Take 5 mL of water in an open china dish and keep it near a window or under a fan.
- Take 5 mL of water in an open china dish and keep it inside a cupboard or on a shelf.
- Record room temperature and time/days taken for evaporation.
- Repeat on a rainy day and record observations.
- What do you infer about the effect of temperature, surface area, and wind velocity on evaporation?
Ans: Observation:
- Water in the china dish near the window/under the fan evaporates fastest.
- Water in the test tube evaporates slower than the china dish due to smaller surface area.
- Water in the cupboard evaporates slowest due to lack of air movement.
- On a rainy day, evaporation is slower in all cases.
Explanation:
- Surface Area: The china dish has a larger surface area than the test tube, allowing more water particles to escape, increasing evaporation rate.
- Temperature: Higher temperatures (assumed near the window) increase particle kinetic energy, speeding up evaporation.
- Wind Velocity: The fan or open window increases air movement, removing water vapor and enhancing evaporation.
- Humidity: On a rainy day, high humidity reduces evaporation as the air is already saturated with water vapor.
|
84 videos|541 docs|60 tests
|
FAQs on NCERT Based Activity: Matter in Our Surroundings - Science Class 9
| 1. What is matter and how is it classified? |  |
| 2. What are the physical properties of matter? |  |
| 3. How does temperature affect the state of matter? |  |
| 4. What is the difference between a physical change and a chemical change? |  |
| 5. Can matter change from one state to another? If so, how? |  |
















