Enzymes Chapter Notes | Biochemistry - NEET PG PDF Download
Overview of Enzymes
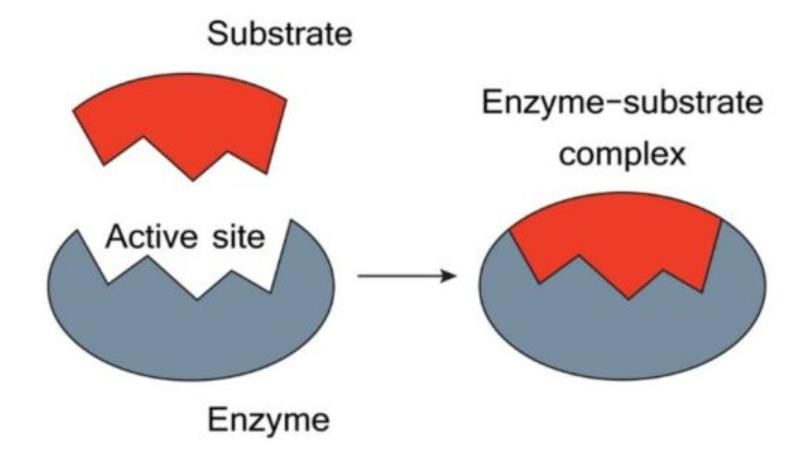
Enzymes are specialized proteins that play a crucial role as catalysts in biological systems. The term "enzyme" was introduced by Frederick Kuhne, originally referring to their activity in yeast.
- Substrate. The substance upon which enzymes act.
- Product. The outcome of the enzymatic action on the substrate.
- Enzymes are sensitive to heat, can be thermally unstable, and are primarily composed of proteins.
- An exception to the protein nature of enzymes is ribozymes.
- Ribozymes are RNA molecules capable of catalyzing biochemical reactions, such as the splicing of pre-mRNA in the spliceosome.
- Ribonuclease P
- Peptidyl Transferase
- RNase H
- Abzymes are antibodies that exhibit catalytic activity.
Types of Enzymes
1. Simple Enzyme
- These enzymes are composed entirely of proteins. They do not have any non-protein components.
2. Complex Enzyme
- Complex enzymes consist of both protein and non-protein parts.
Protein Part: Apoenzyme
- The protein component of a complex enzyme is called the apoenzyme. It is the inactive form of the enzyme.
Non-Protein Part:
- The non-protein part of a complex enzyme can be a prosthetic group, cofactor, or coenzyme.
- Prosthetic Group: These are non-protein organic substances that are thermostable and have a low molecular weight. They are also known as coenzymes.
- Cofactor:. cofactor is a non-protein chemical compound that is necessary for the enzyme's activity.
- Coenzyme:. coenzyme is a type of cofactor that is organic in nature. Coenzymes can attach to enzymes either covalently or noncovalently.
Holoenzyme
- The active form of the enzyme, known as the holoenzyme, is formed when the apoenzyme combines with the non-protein part.
Coenzyme Attachment:
- If a coenzyme is attached to an enzyme covalently, it is called a prosthetic group.
- If the connection between a coenzyme and a substrate is very close, it is referred to as a co-substrate.
In summary, enzymes can be simple or complex, with complex enzymes consisting of a protein part (apoenzyme) and a non-protein part (prosthetic group, cofactor, or coenzyme). The combination of these components determines the enzyme's activity.
Instances of Coenzymes
- Kinases
- ATP. GTP
- Dehydrogenases
- NAD+. FAD
- Pyruvate Dehydrogenase, Alpha Keto Dehydrogenase, Branched Chain Ketoacid Dehydrogenase
- TPP, Lipoic Acid, CoA, FAD
- Transketolase
- Thiamine Pyrophosphate
- Transaminase
- Pyridoxal Phosphate
- Carboxylases
- Biotin
Cofactor and Prosthetic Group
- Prosthetic groups are non-protein components that are firmly attached to the enzyme structure by covalent bonds.
- The most common type of prosthetic group is metals.
- Enzymes with tightly bound metals are referred to as metalloenzymes.
- Cofactors associate reversibly with enzymes or substrates.
- The most common cofactors are various metals.
- Enzymes containing metals as cofactors that are loosely attached are called metal-activated enzymes.
Metal as Cofactors and Prosthetic Group
Zinc: Enzymes that require zinc as a cofactor include:
- Carbonic anhydrase, which helps regulate pH and carbon dioxide levels in the body.
- Carboxypeptidase, involved in protein digestion.
- Alcohol dehydrogenase, important for alcohol metabolism.
- Alkaline phosphatase, which plays a role in removing phosphate groups from molecules.
- ALA dehydratase, involved in heme synthesis.
- Lactate dehydrogenase, important in energy production.
Magnesium: Enzymes that utilize magnesium include:
- Phosphotransferases, such as hexokinase and phosphofructokinase, which are involved in energy metabolism.
- Mutase, an enzyme that facilitates the transfer of a functional group within a molecule.
- Enolase, involved in glycolysis.
- Glucose 6 phosphatase, important for glucose homeostasis.
Copper: Enzymes that require copper include:
- Tyrosinase, involved in melanin production.
- Cytochrome c oxidase, part of the electron transport chain.
- Lysyl oxidase, involved in collagen and elastin cross-linking.
- Superoxide dismutase, which protects against oxidative stress.
- Amino acid oxidase, involved in amino acid metabolism.
Molybdenum: Enzymes that require molybdenum include:
- Xanthine oxidase, involved in purine metabolism.
- Sulfite oxidase, involved in sulfur amino acid metabolism.
Manganese: Enzymes that utilize manganese include:
- Enolase, as mentioned earlier.
- Arginase, involved in urea cycle.
- Phosphotransferases, similar to those mentioned under magnesium.
- Mitochondrial superoxide dismutase, which protects against oxidative damage in mitochondria.
Iron: Enzymes that require iron include:
- Succinate dehydrogenase, involved in the citric acid cycle and electron transport chain.
Calcium: Enzymes that utilize calcium include:
- Lipase, involved in fat digestion.
- Lecithinase, involved in phospholipid metabolism.
IUBMB Classification - Enzyme Classes
The IUBMB classification system categorizes enzymes into six main classes based on the type of reaction they catalyze.
I. Oxidoreductases
- These enzymes are involved in oxidation-reduction reactions, where one substance is oxidized while another is reduced.
II. Transferases
- Transferases are responsible for transferring a functional group, other than hydrogen, from one substance to another.
III. Hydrolases
- Hydrolases catalyze the breakdown of covalent bonds such as C—C, C—O, and C—N through the process of hydrolysis.
- Examples of hydrolases include:
- Pepsin
- Esterases
IV. Lyases
- Lyases are involved in breaking down covalent bonds like C—C, C—O, and C—N by removing atoms and forming double bonds.
- Examples of lyases include:
- Aldolase
- Fumarase
- HMG CoA Lyase
- Argininosuccinate synthetase
V. Isomerases
- Isomerases facilitate geometric or structural changes within a molecule.
- Examples of isomerases include:
- Phosphohexose isomerase
- Racemase
VI. Ligases
- Ligases join two molecules together in reactions that are linked to ATP hydrolysis.
- Examples of ligases include:
- Acetyl CoA carboxylase
- Argininosuccinate synthetase
- PRPP synthetase
- Carbamoyl phosphate synthetase
- Glutamine synthetase
Oxidoreductases
Subclassified into
- Oxygenase
- Monooxygenase
- Dioxygenase
- Catalase
- Peroxidase
- These enzymes are involved in the removal of hydrogen from the substrate.
- They are characterized by their inability to use oxygen as a hydrogen acceptor.
Examples are:
- Succinate Dehydrogenase
Oxidases
- Oxidases play a crucial role in oxidation reactions where oxygen acts as the electron acceptor.
- Some examples of oxidases include:
- Mono Amino Oxidase
- Cytochrome C Oxidase
- Xanthine Oxidase
Oxygenases
- Oxygenases are enzymes that facilitate the direct transfer and incorporation of oxygen into a substrate molecule.
Monooxygenases (Mixed Function Oxidases or Hydroxylases)
- These enzymes incorporate one atom of molecular oxygen into the substrate.
- Examples include:
- Phenylalanine Hydroxylase
- Tyrosine Hydroxylase
- Tryptophan Hydroxylase
- 7 alpha Hydroxylase
- Cytochrome P450, a crucial enzyme in drug metabolism and the production of various bioactive compounds.
Dioxygenases
- These enzymes incorporate both atoms of molecular oxygen into the substrate. The basic reaction can be represented as:
- A + O2 → AO2
Examples of Dioxygenases
- Homogentisate Oxidase
- Tryptophan Pyrrolase (a type of dioxygenase)
- Nitric Oxide Synthase
- Dioxygenases are a subset of oxidoreductases that facilitate the incorporation of oxygen into substrates.
Mechanism of Enzyme Action
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They do this by lowering the activation energy required for the reaction to occur. Activation energy is the energy needed to transform all molecules of a reacting substance from their initial state to the transition state, where the reaction can take place.
Theories Explaining Enzyme Action
Several theories explain how enzymes facilitate reactions:
- Michaelis-Menten Theory. This theory describes the rate of enzyme-catalyzed reactions and how enzymes interact with substrates to form products.
- Fischer's Template Theory. Proposed by Emil Fischer, this theory suggests that enzymes and substrates fit together like a key and lock, with the enzyme acting as a template for the substrate.
- Koshland's Induced Fit Theory. This theory, proposed by Daniel Koshland, emphasizes that the binding of a substrate to an enzyme induces a change in the enzyme's shape, enhancing its ability to catalyze the reaction.
Michaelis-Menten Theory (Enzyme-Substrate Complex Theory)
- An enzyme binds with a substrate to form a temporary enzyme-substrate complex.
- This complex swiftly disintegrates into the enzyme and the products.
Fischer’s Template Theory
- Also referred to as the lock and key model.
- In this model, the three-dimensional shape of the active site in the unbound enzyme is a perfect fit for the substrate.
- However, this theory falls short in explaining the changes that occur during the process of catalysis.
Koshland’s Induced Fit Theory
- According to this theory, when the substrate binds to a specific region of the enzyme, it induces changes in the active site.
- The enzyme undergoes a conformational change either during or after the binding of the substrate.
- This theory effectively explains the alterations that take place during the catalytic process.
Factors Influencing Enzyme Activity
Temperature
- As the temperature increases, it accelerates the speed of both uncatalyzed and enzyme-assisted reactions by enhancing the kinetic energy and the rate of collisions between the reacting molecules.
- When plotting temperature against reaction speed, a bell-shaped curve is observed.
- Many human enzymes begin to denature at temperatures exceeding 40°C, although this can vary depending on the specific enzyme.
- Human enzymes typically remain stable within the range of 45°C to 55°C.
Temperature Coefficient (Q10)
- The temperature coefficient (Q10) measures how much the rate of a biological process increases with a 10°C rise in temperature.
- In most cases, the rates of biological processes can double with just a 10°C increase (Q10 = 2).
Hydrogen Ion Concentration
- The rate of almost all reactions facilitated by enzymes is heavily influenced by the concentration of hydrogen ions (pH).
- Most enzymes found within cells operate optimally at pH levels ranging from 5 to 9.
- The relationship between enzyme activity and hydrogen ion concentration is often illustrated by a bell-shaped curve.
Enzyme Concentration
- At the outset, the speed of an enzyme-catalyzed reaction is directly proportional to the concentration of the enzyme.
Substrate Concentration
- When the concentration of enzymes remains constant, increasing the substrate concentration leads to a rise in the reaction rate.
- This increase in reaction rate continues only up to a certain level of substrate concentration.
- Beyond this specific point, the reaction velocity reaches a maximum and does not increase further, regardless of additional substrate.
Michaelis-Menten Equation
- Vi. (Vmax * S) / (Km + S)
- Vi represents the initial velocity of the reaction.
- Vmax indicates the maximum velocity achievable by the reaction.
- Km is known as the Michaelis constant, a key parameter in enzyme kinetics.
- S denotes the concentration of the substrate involved in the reaction.
Michaelis Constant (Km)
The Michaelis Constant, denoted as Km, refers to the substrate concentration required to achieve half of the maximum reaction velocity (½ Vmax). It is a crucial parameter in enzyme kinetics, providing insights into the affinity between an enzyme and its substrate.
Characteristics of Michaelis Constant
- Independence from Enzyme Concentration: Km is not affected by the concentration of the enzyme.
- Uniqueness: Km is specific to each enzyme-substrate pair, indicating a unique relationship between them.
- Consistency: Km is a constant for a given enzyme-substrate pair, which is why it is considered the signature of the enzyme.
- Affinity Indicator: Km reflects the affinity of the enzyme for its substrate. A lower Km value indicates a higher affinity.
- Natural Substrate Identification: Km aids in identifying the natural substrate of an enzyme. Substrates with a lower Km are likely to be the natural substrates.
Lineweaver-Burk Plot
The Lineweaver-Burk Plot is a graphical method used to represent enzyme kinetics. In this plot, 1/V is shown on the y-axis and 1/S on the x-axis. It is also known as the Double Reciprocal Plot.
Intercepts in the Lineweaver-Burk Plot
- X intercept: -1/Km
- Y intercept: Km/Vmax
- Slope: Km/Vmax
Dixon Plot
- The Dixon Plot is another method to study enzyme kinetics. In this plot, 1/V is measured at different concentrations of inhibitor [I] while keeping the substrate concentration [S] constant.
Types of Enzyme Inhibition
- Competitive Inhibition: This occurs when an inhibitor competes with the substrate for the active site of the enzyme. It can be overcome by increasing the substrate concentration.
- Noncompetitive Inhibition: In this type of inhibition, the inhibitor binds to an allosteric site on the enzyme, regardless of whether the substrate is present or not. This type of inhibition cannot be overcome by increasing the substrate concentration.
- Suicide Inhibition: Also known as mechanism-based inhibition, this occurs when an inhibitor is converted into a reactive form by the enzyme, leading to the irreversible inhibition of the enzyme.
- Allosteric Inhibition: In this type of inhibition, the inhibitor binds to an allosteric site on the enzyme, causing a conformational change that reduces the enzyme's activity.
- Feedback Inhibition: This is a regulatory mechanism in which the end product of a metabolic pathway inhibits an enzyme involved in the pathway, thereby regulating the pathway's activity.
Competitive Inhibition
Competitive inhibition is a phenomenon where an inhibitor competes with a normal substrate for the enzyme's substrate binding site. In this type of inhibition, the inhibitor is a structural analogue of the substrate, meaning it has a similar structure to the substrate.
Features of Competitive Inhibition
- The inhibitor is a structural analogue of the substrate.
- Competitive inhibition is reversible.
- Excess substrate can eliminate the inhibition caused by the inhibitor.
- In competitive inhibition, Vmax remains the same, but Km increases.
Examples of Competitive Inhibition
Competitive Inhibitors That Are Not Drugs
- Oxamate
- Aconitase
- Cisaconitate
- Transaconitate
- Malonate
Noncompetitive Inhibition
- Noncompetitive inhibition occurs when an inhibitor binds to a site on the enzyme that is different from the site where the substrate binds.
- There are two main types of noncompetitive inhibition:
- Reversible Noncompetitive Inhibition. This type of inhibition can be reversed, although only a few noncompetitive inhibitions are reversible.
- Irreversible Noncompetitive Inhibition. In this type, the noncompetitive inhibition cannot be reversed, which is the case for most noncompetitive inhibitions.
Characteristics of Noncompetitive Inhibition
- In noncompetitive inhibition, inhibitors do not structurally resemble the substrate.
- Most instances of noncompetitive inhibition are irreversible, although there are a few reversible cases.
- Adding more substrate does not alleviate the inhibition.
- The Km value remains constant.
- Vmax decreases.
Instances of Irreversible Noncompetitive Inhibition
- Irreversible noncompetitive inhibitors are commonly toxic agents.
Noncompetitive Inhibitor:
- Cyanide
- Iodoacetate
- Glyceraldehyde 3 Phosphate
- Fluoride
- Disulfiram (Antabuse)
- Aldehyde Dehydrogenase
- British Anti-Lewisite (Dimercaprol). impacts the -SH group of various enzymes
- Arsenite
- Alpha Ketoglutarate Dehydrogenase
- Fluoroacetate
- Diisopropyl Fluorophosphate
- Serine Proteases
- Certain inhibitors of the electron transport chain also exemplify irreversible noncompetitive inhibition.
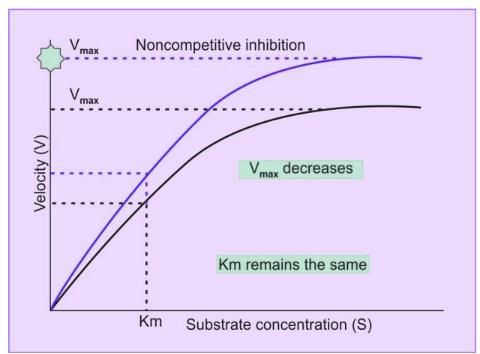 Noncompetitive inhibition
Noncompetitive inhibition
Suicide Inhibition: An Overview
- Suicide inhibition, also referred to as mechanism-based inactivation, is a specific type of irreversible inhibition.
- In this process, inhibitors are initially unreactive until they bind to the active site of a particular enzyme.
- Once the inhibitor attaches to the enzyme, it undergoes a transformation into a potent inhibitor through the enzyme's catalytic action.
- This transformed inhibitor then binds irreversibly to the enzyme, effectively blocking its activity.
Instances of Suicide Inhibition
- Allopurinol is a medication that inhibits Xanthine Oxidase, an enzyme involved in purine metabolism.
- In the body, allopurinol is converted into alloxanthine, which irreversibly inhibits Xanthine Oxidase, reducing uric acid production.
- Difluoromethylornithine (DFMO) is used in the treatment of Trypanosomiasis, a parasitic disease. DFMO acts as a suicide inhibitor of Ornithine Decarboxylase, an enzyme crucial for polyamine synthesis, thereby disrupting the growth of the parasite.
- Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) that acetylates the active site of Cyclooxygenase (COX) enzymes. This modification inhibits the production of prostaglandins, which are mediators of inflammation, pain, and fever.
Feedback Inhibition
Feedback inhibition, also called end-product inhibition, is a regulatory mechanism in metabolic pathways. In this process, the final product of a biochemical pathway inhibits an earlier step in the pathway, thereby reducing the activity of the enzyme responsible for that step. This mechanism helps to prevent the overproduction of the end product and maintains metabolic balance. For instance, in purine synthesis, AMP acts as an inhibitor by feedback inhibition, reducing the activity of the enzyme involved in the initial step of the pathway.
Regulation of Enzymes
Regulation of Enzyme Quality (Intrinsic Catalytic Efficiency):
Allosteric Regulation:Allosteric enzymes play a crucial role in regulating metabolic pathways. These enzymes have two distinct sites: a catalytic site where the substrate binds and a regulatory site where a modifier can bind. Depending on the modifier's effect, allosteric regulation can be classified into:
- Allosteric Inhibition: When the modifier binds to the regulatory site and inhibits the enzyme's activity, it is called allosteric inhibition. This type of regulation helps to decrease the enzyme's activity and regulate the metabolic pathway.
- Allosteric Activation: Conversely, when the modifier binds to the regulatory site and enhances the enzyme's activity, it is known as allosteric activation. This type of regulation increases the enzyme's catalytic efficiency and promotes the metabolic pathway.
Covalent Modification: Covalent modification involves the addition or removal of specific chemical groups to or from the enzyme, leading to changes in its activity. This type of regulation can either activate or deactivate the enzyme, depending on the nature of the modification. Common examples of covalent modification include phosphorylation, acetylation, and methylation, which play essential roles in regulating enzyme activity and metabolic pathways.
Regulation of Enzyme Quantity:
- Control of Enzyme Synthesis: Enzyme quantity can be regulated by controlling the synthesis of enzymes through induction and repression mechanisms. Induction involves the activation of gene expression to produce specific enzymes in response to certain signals or conditions. Repression, on the other hand, involves the suppression of gene expression to prevent the synthesis of specific enzymes when they are not needed.
- Control of Enzyme Degradation: Enzyme degradation is another mechanism to regulate enzyme quantity. By controlling the degradation of enzymes, cells can determine the levels of specific enzymes present in the system. This can be achieved through various cellular processes, such as ubiquitination, where enzymes are tagged for degradation by proteasomes, thereby regulating enzyme levels and activity in the cell.
- Additional Mechanisms: Apart from allosteric regulation, enzyme quantity can also be managed through various other mechanisms tailored to specific cellular needs and conditions. These mechanisms ensure that the levels and activities of enzymes are maintained at optimal levels for efficient metabolic functioning.
Features of Allosteric Regulation
- The modifier involved in allosteric regulation does not have to be a structural analogue of the substrate. This means that the modifier can be different in structure from the substrate.
- Allosteric regulation can be considered reversible to some extent if there is an excess of substrate present.
- Most allosteric enzymes have a quaternary structure and are made up of multiple subunits.
- Allosteric enzymes are essential in metabolic pathways and are often referred to as regulatory enzymes due to their crucial roles.
- Hill's Equation is used to explain the behavior of enzymes that exhibit cooperative binding of the substrate.
- The sigmoid shape of the curve observed in cooperative binding is a result of the cooperative nature of substrate binding.
- Cooperative Binding refers to the phenomenon where the binding of a substrate to one site on a subunit increases the likelihood of substrate binding to other sites on different subunits.
Two Categories of Allosteric Enzymes
- K Series: In this type, the Km value can change based on the enzyme and the specific conditions, while the Vmax remains constant.
- V Series: Here, the Vmax decreases, but the Km value stays the same.
Similarities between Allosteric Regulation and Noncompetitive Inhibition
- In both cases, the modifier or inhibitor is not a structural analogue of the substrate.
- The modifier or inhibitor binds to a site that is different from the substrate binding site in both scenarios.
- In the V series of allosteric enzymes and in noncompetitive inhibition, the Km value remains the same while the Vmax decreases.
Differences between Noncompetitive and Allosteric Inhibition
- Noncompetitive Inhibition: When substrate concentration is varied, the effect on enzyme activity produces a hyperbolic curve.
- Allosteric Inhibition: In contrast, varying substrate concentration in allosteric inhibition results in a sigmoid curve.
Examples of Allosteric Enzymes
- Activators: ALA Synthase and Heme.
- Aspartate Transcarbamoylase
- CTP
- Cholesterol
- Fructose 2,6-bisphosphate
- Citrate
- Pyruvate Carboxylase
- ADP
- Acetyl CoA Carboxylase
- Acyl CoA
- Citrate Synthase
- Carbamoyl Phosphate
- Synthetase I
- N Acetyl
- Glutamate (NAG)
- Synthetase II
- UTP
Covalent Modification of Enzyme Activity
Covalent modification is a method used to regulate enzyme activity by adding or removing a group through the formation or breaking of a covalent bond. This process can either enhance or diminish enzyme activity, depending on the specific modification.
Types of Covalent Modification
- Irreversible Covalent Modification. This involves partial proteolysis or zymogen activation, where a covalent bond is permanently altered, leading to a permanent change in enzyme activity.
- Reversible Covalent Modification. This type involves the addition or removal of a specific group, allowing for temporary changes in enzyme activity.
Common Methods of Reversible Covalent Modification
- Phosphorylation. Dephosphorylation. This is the most common type of covalent modification, where a phosphate group is added or removed, leading to changes in enzyme activity.
- Methylation. The addition of a methyl group can alter enzyme activity by changing the enzyme's structure or function.
- Adenylation. This involves the addition of an adenyl group, which can impact enzyme activity by altering its conformation or function.
- ADP Ribosylation. The addition of an ADP ribose group can modify enzyme activity by changing its structure or function.
- Acetylation. The addition of an acetyl group can influence enzyme activity by altering its conformation or function.
Examples of Covalent Modifications
- When the body is fasting and influenced by glucagon, enzymes are typically in a phosphorylatedstate. The enzymes that are active in this state include:
- Glycogen Phosphorylase
- Key enzymes of Gluconeogenesis
- Citrate Lyase
- Phosphorylase Kinase
- HMG CoA Reductase Kinase
- On the other hand, when the body is in a well-fed state and influenced by insulin, enzymes are in a dephosphorylatedstate. The enzymes that are active in this state include:
- Glycogen synthase
- Key enzymes of Glycolysis
- Pyruvate dehydrogenase
- HMG CoA reductase
Protein Acetylation: A Common Covalent Modification of Metabolic Enzymes
- Acetylation is a modification where an acetyl group is added to a protein, and it can occur in many mammalian proteins, including nearly all enzymes involved in crucial metabolic pathways.
- This process, known as acetylation-deacetylation, affects numerous proteins within a pathway, setting it apart from other covalent modifications.
- The degree of acetylation in metabolic enzymes is primarily determined by the energy status of the cell.
- In cells with abundant nutrients, high levels of Acetyl CoA encourage lysine acetylation. Conversely, when nutrients are scarce, Acetyl CoA levels drop, and the ratio of NAD+ to NADH rises, promoting protein deacetylation.
- Lysine acetyltransferases facilitate the transfer of an acetyl group from acetyl-CoA to the ε-amino groups of lysine residues, resulting in the formation of N-acetyl lysine.
- Certain proteins, particularly those in the mitochondria, can undergo acetylation by directly reacting with acetyl-CoA, bypassing the need for an enzyme.
Deacetylation
- There are two main types of protein deacetylases:
- Histone deacetylases remove acetyl groups through hydrolysis, reverting the protein to its unmodified state and producing acetate.
- Sirtuins utilize NAD+ as a substrate, leading to the formation of O-acetyl ADP-ribose and nicotinamide, in addition to the unmodified protein.
What are Isoenzymes?
Isoenzymes, also known as isoforms, are different physical versions of the same enzyme that catalyze the same chemical reaction. These enzymes can vary in their molecular form and can be produced in the same or different tissues.
Characteristics of Isoenzymes
- Isoenzymes are capable of catalyzing the same chemical reactions, but they may differ in certain properties.
- They can vary in heat stability. For example, there are heat-stable and heat-labile forms of alkaline phosphatase (ALP).
- Isoenzymes differ in their electrophoretic mobility, meaning they move at different rates in an electric field. For instance, creatine kinase type I (CK-I) moves faster than creatine kinase type III (CK-3).
- They also respond differently to inhibitors. For example, there are tartaric-labile and tartaric-stable forms of acid phosphatase.
- Isoenzymes have different subunit compositions. For instance, lactate dehydrogenase type 1 (LDH-1) has an H4 subunit composition, while lactate dehydrogenase type 5 (LDH-5) has an M4 subunit composition.
- They are found in different tissues within the body. For example, LDH-1 is predominantly found in the heart, while LDH-5 is found in muscle tissue.
- Isoenzymes can also differ in their Michaelis-Menten constant (Km) values. For instance, glucokinase, an isoenzyme of hexokinase, has a high Km value, whereas hexokinase has a low Km value.
Functional Enzymes and Nonfunctional Enzymes
Functional enzymes are responsible for carrying out specific tasks in the plasma.
Examples of Functional Enzymes
- Coagulation Factors
- Lipoprotein Lipase
These enzymes are released from tissues due to normal wear and tear, and their levels in the serum are usually low. However, when there is tissue damage, the levels of these enzymes increase in the serum, helping to identify the location of tissue injury.
Examples of Nonfunctional Enzymes
- LDH (Lactate Dehydrogenase)
- Creatine Kinase
- Alkaline Phosphatase
Isoenzymes of Lactate Dehydrogenase (LDH)
- LDH has five isoenzymes. It is a tetramer made up of two different types of subunits: H (heart) and M (muscle).
- Isoenzyme Names
- Subunit Composition
- Tissue Localization
- LDH-1
- H4
- Predominantly found in the heart
- LDH-2
- H3M1
- Found in red blood cells (RBC)
- LDH-3
- H2M2
- Found in the brain
- LDH-4
- HM3
- Found in the liver and skeletal muscle
- LDH-5
- MM
- Predominantly found in the liver
- LDHx
- An atypical isoenzyme found in male genital tissues
Isoenzymes of Creatine Kinase (CK)
- CK has three isoenzymes, consisting of two types of subunits: M (muscle) and B (brain) .
- CK-1
- Subunit Composition: BB (brain type)
- CK-2
- Subunit Composition: MM (muscle type)
Two Unusual Types of Creatine Kinase
- CK Macro (Macro–CK): This type is created when CK-BB combines with antibodies such as IgG.
- CK-Mi (Mitochondrial CK): This enzyme is found on the outer surface of the inner mitochondrial membrane, particularly in muscles, the liver, and the brain.
Isoenzymes of Alkaline Phosphatase (ALP)
- Alpha-1 ALP. Produced by epithelial cells located in the biliary canaliculi.
- Alpha-2 Heat Labile ALP. Secreted by hepatic cells in the liver.
- Alpha-2 Heat Stable ALP. This isoenzyme is generated by the placenta and is inhibited by the amino acid phenylalanine.
- Pre Beta ALP. Synthesized by osteoblasts, which are cells responsible for bone formation.
- Gamma ALP. Produced by intestinal cells in the gastrointestinal tract.
- Leukocyte ALP. This isoenzyme is made by leukocytes, or white blood cells, which are part of the immune system.
- Regan Isoenzyme. Named after the first patient from whom it was isolated, this isoenzyme resembles Alpha-2 Heat Stable ALP. It is also known as the carcino-placental isoenzyme and is typically elevated in cases of lung, liver, and intestinal carcinomas (cancers).
- Nagao Isoenzyme. A variant of the Regan isoenzyme, the Nagao isoenzyme is inhibited by the amino acid L-leucine.
Cardiac Biomarkers
- Creatine Kinase. CKMB]
- Cardiac Troponin I. CTnI]
- Cardiac Troponin T. CTnT]
- Brain Natriuretic Peptide. BNP] : indicates heart failure, but not a sign of Myocardial Infarction.
- Myoglobin
- Lactate Dehydrogenase. LDH] : used less frequently in modern practice.
- Aspartate Aminotransferase. AST] : also used less often in current practice.
Flipped Pattern of LDH
- Under normal circumstances, LDH-2 is found in higher amounts than LDH-1.
- This pattern changes in Myocardial Infarction.
- This alteration has limited value for diagnosis.
New Cardiac Biomarkers
- Ischemia Modified Albumin
- Glycogen Phosphorylase BB Isoenzyme
- Pregnancy Associated Plasma Protein A (PAPP-A)
- Myeloperoxidase (MPO)
Enzyme Profile for Liver Diseases
Enzymes that are elevated in the serum indicate damage to liver cells.
Aminotransferases, also known as transaminases, are crucial markers of liver cell injury.
They are especially useful for diagnosing acute liver conditions such as hepatitis.
- Aspartate aminotransferase (AST) is found in the liver, heart, skeletal muscles, kidneys, brain, pancreas, lungs, white blood cells, and red blood cells.
- Alanine aminotransferase (ALT) is primarily located in the liver, making it a more specific indicator of liver disease compared to AST.
Indicators of Cholestasis
Certain enzymes that are elevated in serum can also suggest cholestasis, including:
- Alkaline phosphatase (ALP)
- 5’-nucleotidase
- γ-glutamyltransferase (GGT)
While GGT is less specific for cholestasis than ALP or 5’-nucleotidase, it can also be useful in identifying hidden alcohol use.
An increase of less than three times the normal level of ALP can occur in various liver diseases, but the degree of increase can vary widely and should not be standardised.
A more than four-fold increase in ALP is indicative of cholestasis.
ALP levels do not help in differentiating between intrahepatic and extrahepatic cholestasis.
5’ Nucleotidase is more specific for cholestasis than ALP and GGT.
Nonpathologic Elevations of ALP
- Patients over 60 years old
- Individuals with Type O and Type B blood groups
- After eating a fatty meal (due to the release of gamma ALP)
- Children undergoing rapid bone growth
- Late stages of normal pregnancy
AST/ALT Ratio
- The AST:ALT ratio is used to indicate conditions causing hepatocellular damage, as AST levels rise above ALT levels in such cases. This is because AST is more specific to hepatocellular damage than ALT.
- Conditions associated with this ratio include:
- Chronic viral hepatitis
- Nonalcoholic fatty liver disease
- Toxic hepatitis
- Paracetamol toxicity
- An AST:ALT ratio > 2:1 suggests alcoholic liver disease, while a ratio > 3:1 is highly indicative of it.
- Aminotransferases (ALT and AST):
- ALT is more specific for hepatocellular damage than AST.
- In hepatocellular disease, ALT elevation is equal to or slightly higher than AST, resulting in an AST/ALT ratio of less than 1.
- If cirrhosis occurs, the ratio becomes greater than 1.
- An ALT elevation of less than 300 IU/L is nonspecific, while a level above 1000 IU/L indicates significant hepatocellular injury.
- In alcoholic liver disease, a deficiency of Pyridoxal Phosphate caused by alcohol reduces transaminase levels (ALT and AST). Consequently, ALT levels are often normal and AST levels rarely exceed 300 IU/L.
Enzyme Profile in Prostate Cancer
- α-Glutathione S-Transferase
- γ-Glutamyltranspeptidase
- β2-Microglobulin
- α-1-Macroglobin
- Retinol Binding Protein
- Cystatin C
- Microalbumin
- Osteopontin
- Liver Fatty Acid Binding Protein
- Sodium–Hydrogen Exchanger Isoform
- Exosomal Fetuin
Enzyme Markers of Prostate Cancer
- Tartrate Labile Acid Phosphatase
- Prostate-Specific Antigen (PSA)
- Also known as Kallikrein related Peptidase 3 (KLK3)
- It is a serine protease
- Secreted by epithelial cells of the prostate
- Specific to prostate, but not specific to prostate cancer
- The usual cut-off for prostate cancer is a PSA level over 4 ng/mL
- No PSA level ensures a zero risk of prostate cancer
- PSA level tests should be followed by a prostate biopsy
Markers of Bone Diseases
- Bone diseases can be assessed by measuring specific markers related to bone formation and resorption.
Bone Formation
- Serum Bone-specific Alkaline Phosphatase (BAP)
- Serum Osteocalcin
- Serum Propeptide of type I Procollagen
Bone Resorption
- Urine and serum cross-linked N-telopeptide
- Urine and serum cross-linked C-telopeptide
- Urine total free deoxypyridinoline
Serine Proteases
Enzyme Profile in Pancreatitis
The following enzymes are relevant:
- Amylase
- A serum lipase level measurement can be useful in distinguishing between a pancreatic and a non-pancreatic cause of hyperamylasemia.
- Measuring serum lipase levels can help differentiate between pancreatic and non-pancreatic causes of hyperamylasemia.
- In addition to serum, amylase levels can also be measured in urine.
Biomarkers of Acute Kidney Injury
- Proteolytic enzymes are a type of enzyme that have serine in their active site.
- These enzymes play a crucial role in various biological processes by breaking down proteins.
- The active site of serine proteases, a specific group of proteolytic enzymes, contains a triad of amino acids: serine, histidine, and aspartate.
Examples of Serine Proteases
- Chymotrypsin: An enzyme that helps in the digestion of proteins in the small intestine.
- Trypsin: Another digestive enzyme that plays a role in breaking down proteins in the small intestine.
- Elastase: An enzyme involved in the breakdown of elastin, a protein that helps maintain the elasticity of tissues.
- Thrombin: An enzyme that plays a critical role in blood coagulation by converting fibrinogen to fibrin.
- Plasmin: An enzyme involved in the breakdown of fibrin in blood clots, helping to dissolve clots after they are no longer needed.
- Complement proteins:. group of proteins that play a role in the immune response by marking pathogens for destruction.
- Factor X and XI: Proteins involved in the coagulation cascade, playing a role in blood clot formation.
- Prostate Specific Antigen (PSA):. protein produced by the prostate gland, elevated levels of which can indicate prostate issues.
Novel Biomarkers of Acute Kidney Injury
- Kidney Injury Molecule-1 (KIM-1):. protein that is upregulated in response to kidney injury and can be detected in urine.
- Neutrophil Gelatinase-associated Lipocalin (NGAL):. protein that increases in response to kidney injury and can be measured in urine or blood.
- IL-18: An inflammatory cytokine that can be elevated in cases of acute kidney injury.
- Alanine Amino Peptidase: An enzyme that can be elevated in urine following kidney injury.
- Clusterin:. protein that can be involved in the response to kidney injury and may be elevated in urine.
Differences in Substrate Specificity Among Serine Proteases
- Trypsin is specific for cleaving basic amino acids.
- Chymotrypsin targets bulky hydrophobic amino acids for cleavage.
- Elastase preferentially cleaves small neutral amino acids such as Alanine and Glycine.
Bi-Bi Reaction
- The kinetic behavior of two-substrate, two-product reactions is referred to as Bi-Bi reactions.
- Most Bi-Bi reactions adhere to Michaelis-Menten kinetics.
Types of Bi-Bi Reactions
- Ordered Bi-Bi Reaction: This type includes reactions such as NAD(P)H-dependent oxidoreductases.
- Random Bi-Bi Reaction: Many kinases and some dehydrogenases fall under this category.
- Ping-Pong Reaction: This includes enzymes like aminotransferases and serine proteases.
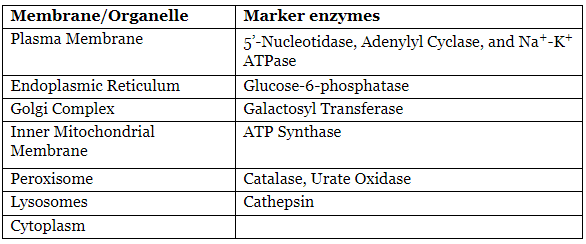
Enzymes as Markers of Organelles and Membranes
Enzymes as Diagnostic Reagents
- Diagnostic Test
- Urease
- Urea Estimation
- Uricase
- Uric Acid Estimation
- Glucose Oxidase
- Peroxidase
- Glucose, Cholesterol
- Cholesterol Oxidase
- Creatininase
- Creatinine
- Triglycerides
Enzymes in Other Body Fluids
- Clinical Use
- Lactate Dehydrogenase in CSF, pleural fluid, and ascitic fluid
- This finding suggests a malignant tumor but is not definitive.
- Adenosine Deaminase in pleural fluid
- This finding indicates a tuberculous pleural effusion.
- Amylase in urine
- This finding suggests pancreatitis.
|
50 docs|7 tests
|
FAQs on Enzymes Chapter Notes - Biochemistry - NEET PG
| 1. What are enzymes and how do they function in biochemical reactions? |  |
| 2. What is the difference between coenzymes and cofactors? |  |
| 3. How do metal ions function as cofactors in enzymatic reactions? |  |
| 4. What is the role of oxidoreductases in biochemical processes? |  |
| 5. How do enzymes lower activation energy, and why is this important? |  |




















