Chemistry of Carbohydrates Chapter Notes | Biochemistry - NEET PG PDF Download
Introduction
- Carbohydrates are the most abundant organic molecules in nature. The word 'carbohydrate' refers to a compound made up of carbon and water.
- Definition: Carbohydrates are substances that, when hydrolyzed, yield aldehyde or keto derivatives of polyhydric alcohols.
- General Formula: The general formula for carbohydrates is Cₙ(H₂O)ₙ, where 'n' represents the number of carbon atoms.
Number of Carbon Atoms: Carbohydrates are classified based on the number of carbon atoms present:
- 3 Carbon Atoms - Trioses
- 4 Carbon Atoms - Tetroses
- 5 Carbon Atoms - Pentoses
- 6 Carbon Atoms - Hexoses
- 7 Carbon Atoms - Heptoses
- 9 Carbon Atoms - Nonoses
Role in Human Nutrition: Carbohydrates, especially glycogen, are the primary source of energy for humans, providing about 45-65% of total energy intake.
Classification of Carbohydrates
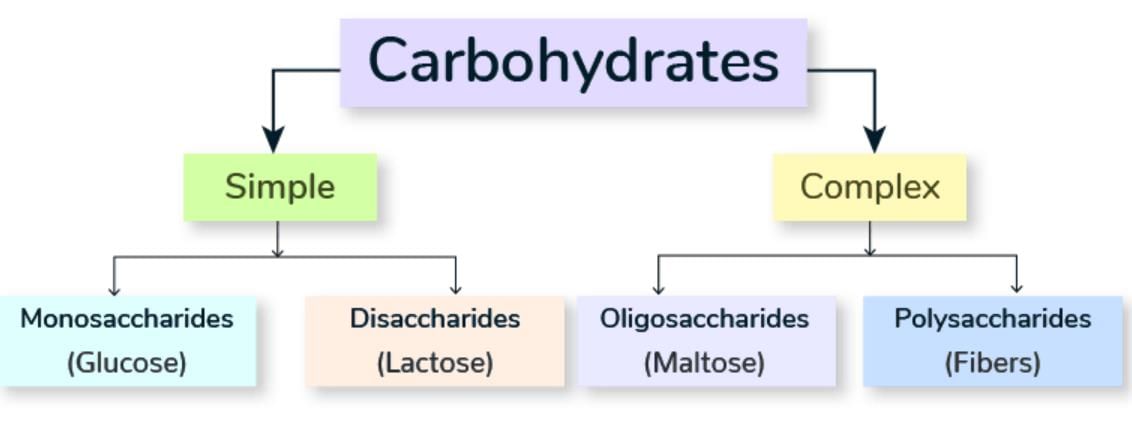
Carbohydrates can be classified into:
- Monosaccharides
- Disaccharides
- Oligosaccharides
- Polysaccharides
Monosaccharides [Cn(H2O)n]
Monosaccharides are the most basic form of carbohydrates and are often referred to as simple sugars. They cannot be further broken down into smaller sugar units. These sugars consist of a single sugar unit and are the fundamental building blocks of all carbohydrates.
Classification Based on Functional Group:
- Aldoses: Monosaccharides with an aldehyde group.
- Ketoses: Monosaccharides with a keto group.
Important Monosaccharides:
- Glyceraldehyde:. simple sugar with an aldehyde group.
- Dihydroxyacetone:. simple sugar with a keto group.
- Erythrose:. four-carbon sugar (tetrose) with an aldehyde group.
- Erythrulose:. four-carbon sugar with a keto group.
- Ribose:. five-carbon sugar (pentose) with an aldehyde group, essential for RNA.
- Xylose: An epimer of Ribose, differing in the configuration around one carbon atom. Arabinose: Another five-carbon sugar with an aldehyde group.
- Ribulose:. five-carbon sugar with a keto group.
- Xylulose: An epimer of Ribulose.
- Glucose:. six-carbon sugar (hexose) with an aldehyde group, a primary energy source for cells.
- Galactose:. hexose sugar, an epimer of Glucose, important in lactose.
- Mannose: Another hexose sugar, an epimer of Glucose, found in fruits.
- Fructose:. hexose sugar with a keto group, found in many fruits and honey.
- Sedoheptulose:. seven-carbon sugar (heptose) with a keto group.
Key Characteristics of Monosaccharides
- Simplest Carbohydrates: Glyceraldehyde and Dihydroxyacetone are the most basic and simplest forms of carbohydrates that are of biological interest.
- Pentoses in Nucleic Acids: Ribose and Deoxyribose are pentose sugars that are integral parts of nucleic acids like RNA and DNA.
- Biologically Significant Nanos: Neuraminic Acid is an example of a nanos (nine-carbon sugar) that has biological significance.
Sialic Acid
- N-Acyl or O-Acyl derivative of Neuraminic Acid
- N-Acetyl Neuraminic Acid (NANA) is the main type of sialic acid.
- They are parts of both glycoproteins and gangliosides.
Ring Structures of Monosaccharides
- Monosaccharides with 4, 5, or 6 carbon atoms can easily bend and twist.
- This bending helps the aldehyde or keto group get close to other hydroxyl groups on the same sugar molecule.
- When a ketone joins with a hydroxyl group, it creates a hemiketal ring.
- Similarly, when an aldehyde joins with a hydroxyl group, it forms a hemiacetal ring.
- If the ring has six sides, made up of 5 carbon atoms and 1 oxygen atom, it's called a pyranose ring.
- If the ring has five sides, consisting of 4 carbon atoms and 1 oxygen atom, it's known as a furanose ring.
- For instance, fructose typically forms a furanose ring, known as fructofuranose. On the other hand, glucose usually exists as a pyranose ring, referred to as glucopyranose.
Biologically Important Hexoses
- Glucose is the primary sugar present in the human body.
- It is the main source of metabolic energy for mammals.
- Glucose is also known as Dextrose because it is dextrorotatory, as per the QNBE model (2012).
- Glucose serves as the universal fuel for the fetus.
- The following organs predominantly depend on glucosefor energy:
- Brain
- Renal medulla
- Cornea
- Retina
- Testis
- Red blood cells (RBC)
- It is important to highlight that glucose is a vital metabolic fuel for mature erythrocytes, whether in a fed or fasting state, as noted by AIIMS in May 2015.
- Glucose is a constituent of lactose (milk sugar).
- It is synthesized in the mammary gland during lactose production.
- Glucose is also found in glycoproteins, glycosaminoglycans in proteoglycans, and glycolipids.
- It can be extracted from plant mannans, which is how it acquired its name.
- Glucose is present in glycoproteins and mucoproteins.
- It is a component of sucrose, the common sugar.
- Glucose is found in fruit juices, honey, and sugarcane.
- It is also present in seminal fluid.
- All hexoses have a free functional group, making them reducing sugars.
Disaccharides
Disaccharides are formed when two monosaccharide units are joined by a glycosidic bond or can form two monosaccharide units through hydrolysis. They are categorized based on their reducing ability.
- Nonreducing disaccharides: These disaccharides have their functional groups involved in forming the glycosidic bond, making them unavailable for reduction.
- Reducing disaccharides: These disaccharides have free functional groups that are available for reduction.
Reducing Disaccharides with Free Functional Groups
Maltose:
- Composed of two α-D-Glucose units.
- Linkage: α 1→4 linkage.
Isomaltose:
- Composed of two α-D-Glucose units.
- Linkage: α 1→6 linkage.
Lactose (Milk Sugar):
- Composed of D-Galactose and β-D-Glucose.
- Linkage: β 1→4 linkage.
Lactulose:
- Composed of α-D-Galactose and β-D-Fructose.
- Linkage: α 1→β 4 linkage.
Non-reducing Disaccharides — No Free Functional Group
- Trehalose. Sugar found in the hemolymph of insects, yeast, and fungi )
- α1 → α1 Linkage
- Sucrose. Cane Sugar )
- α-D-Glucose. β-D-Fructose
- α1 → β2 Linkage
- It acts as an osmotic laxative.
- Mainly synthetic ( small amount found in heated milk )
- This substance is not hydrolysed by intestinal bacteria. However, it is fermented by intestinal bacteria.
Oligosaccharides
- Oligosaccharides are formed by the condensation of 3 to 10 monosaccharides, resulting in 3–10 monosaccharide units upon hydrolysis.
- Blood group substances are a type of oligosaccharide.
- Oligosaccharides are typically found in association with proteins (in the form of glycoproteins and proteoglycans) and lipids (as glycolipids).
Polysaccharides
Polysaccharides are complex carbohydrates formed by the condensation of more than 10 monosaccharide units or by releasing more than 10 monosaccharide units during hydrolysis. They are also referred to as glycans.
Polysaccharides are classified into two main types based on the types of monosaccharide units they contain:
- Homoglycans (or Homopolysaccharides): These consist of only one type of monosaccharide unit.
- Heteropolysaccharides (or Heteroglycans): These contain different types of monosaccharide units.
Glycogen, a polysaccharide, serves as the storage form of glucose in animals, commonly known as animal starch.
- Glycogen is composed of α-D glucose units.
- It is structured as a branched polymer of glucose.
- The linear sections of glycogen feature α 1,4 linkages, while the branches are connected by α 1,6 linkages.
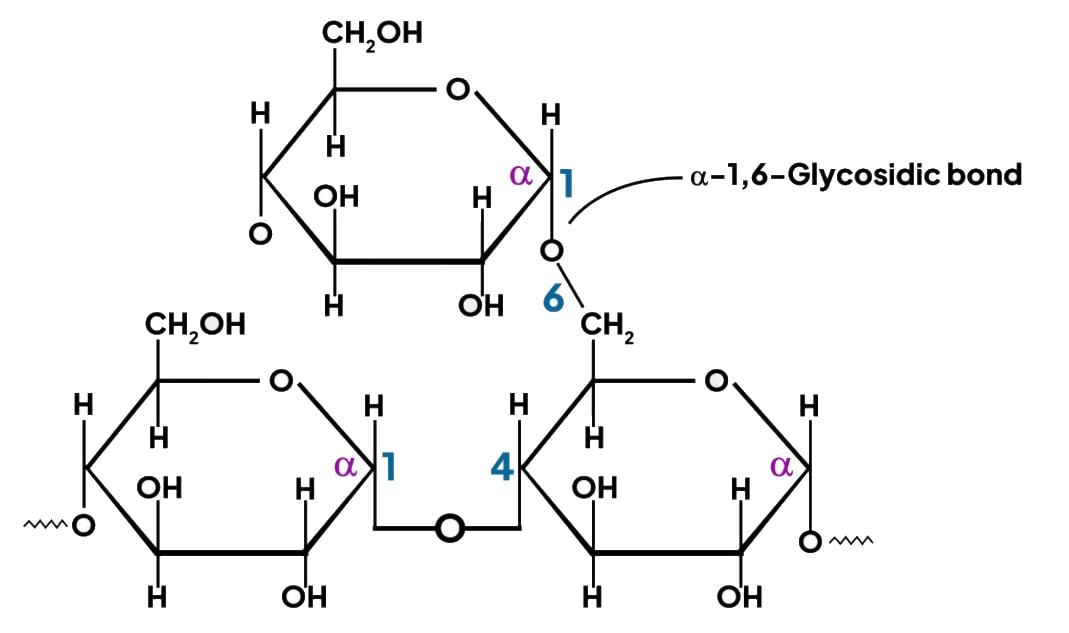 Structure of glycogen
Structure of glycogen
Muscle Glycogen
- Muscle glycogen contains granules known as β particles, each containing approximately 60,000 glucose residues.
Liver Glycogen
- In addition to β particles, liver glycogen also includes rosettes of glycogen granules, which are clusters of β particles.
Starch
- Starch is how plants store carbohydrates.
- It is a type of homopolysaccharide made only of glucose.
- Starch is also called glucan.
- The two main parts of starch are:
- Amylose (about 20–30%) which has a helical structure that does not branch.
- Amylopectin (80–87%) which contains branched chains with 24–30 glucose units.
- Starch is a mixture of:
- Amylose: 15–20%
- Amylopectin: 80–85%
- Amylose
- Soluble and unbranched
- Has α-1,4 linkages
- Amylopectin
- Insoluble and branched
- Contains α-1,4 and α-1,6 linkages
Chitin
- Chitin is found in the exoskeletons of crustaceans and insects, as well as in the cell walls of fungi like mushrooms.
- It is made up of N-acetyl-D-glucosamine units that are connected by β-1 → 4 glycosidic bonds.
Cellulose
- Cellulose is the main component of the plant cell wall.
- It is a homopolysaccharide composed of β-glucose molecules linked together by β-1,4 bonds.
- Cellulose is a significant part of dietary fibre, contributing to the bulk of food.
- Humans are unable to digest cellulose because they do not have the necessary enzymes to break it down.
Inulin (Fructosan)
- Inulin is a homopolysaccharide made up of fructose units linked by β-2 → 1 bonds.
- It is found in the roots of plants such as dahlias, chicory, onion, garlic, and dandelions.
- Inulin is classified as a type of fibre and is soluble in water.
- It is used in medical tests to measure glomerular filtration rate (GFR) because it is not broken down by human digestive enzymes.
Dextran
- Dextran is a polysaccharide composed of α-glucose molecules linked in various ways, including α 1, 6; α 1, 4; and α 1, 3 linkages.
- It is used as a plasma volume expander in medical settings.
Plasma Expanders
- Plasma expanders are high molecular weight substances that create colloid osmotic pressure, helping to keep fluid within blood vessels.
- Common plasma expanders include:
- Human albumin
- Hydroxyethyl starch (Hetastarch)
- Degraded gelatin polymer
Compare and Study
- Dextrose is the clinical term for dextrorotatory glucose.
- Dextrin are products formed during the breakdown of starch, both chemically and enzymatically.
- Homopolysaccharide consisting of Glucose.
- Disaccharide made up of Galactose and Glucose.
- Lactase is the enzyme that breaks down lactose into Galactose and Glucose.
- Lactate is produced from pyruvate during anaerobic respiration.
- Disaccharide made up of Galactose and Fructose.
Heteropolysaccharides (Heteroglycans)
Glycosaminoglycans (Mucopolysaccharides)
- Pectins
- Agarose
- Agar
Mucopolysaccharides (Glycosaminoglycans)
- Glycosaminoglycans consist of straight chains of hetero-polysaccharides formed by repeating disaccharide units.
- Each disaccharide unit is composed of an aminosugar and a uronic acid.
- These molecules were initially discovered in mucin, which is why they are referred to as mucopolysaccharides.
- Glycosaminoglycans play a crucial role in the structure of the extracellular matrix.
Properties of GAG
- The presence of numerous negative charges (such as COO–, acetyl, and sulfate groups) causes these chains to repel each other.
- This repulsion is responsible for the slippery consistency of mucus and synovial fluid.
- When water is expelled, these chains occupy less space, but when pressure is alleviated, they revert to their original size due to the repulsive forces between the negative charges.
- This property accounts for the resilient nature of synovial fluid and vitreous humour.
- Glycosaminoglycans have the capacity to retain large amounts of water, making them essential components of the extracellular matrix.
- Disaccharide repeat units are characteristic of glycosaminoglycans (GAG).
Biologically Significant Glycosaminoglycans (GAGs)
Hyaluronic Acid (Hyaluronan)
- Disaccharide Repeat Unit: Present in Skin, Synovial fluid, Bone, Cartilage, Vitreous humour, Loose connective tissue, and Umbilical cord.
Chondroitin Sulfate
- Disaccharide Repeat Unit: Found in Cartilage, Bone, and Central nervous system (CNS).
Keratan Sulfate Types I and II
- Disaccharide Repeat Unit: Located in Loose connective tissue.
Heparin
- Disaccharide Repeat Unit: Found in Mast cells, Liver, Lung, and Skin.
Heparan Sulfate (HS)
- Disaccharide Repeat Unit: Present in Kidney basement membrane.
Dermatan Sulfate (DS)
- Disaccharide Repeat Unit: Found in Wide distribution.
Glycosaminoglycans
Glycosaminoglycans (GAGs) are long, unbranched polysaccharides consisting of repeating disaccharide units. These disaccharides typically contain one amino sugar (such as glucosamine or galactosamine) and one uronic acid (such as glucuronic acid or iduronic acid). GAGs are negatively charged due to the presence of sulfate groups and carboxyl groups, which attract water and create a gel-like consistency. This property makes GAGs essential for maintaining tissue hydration and providing structural support. GAGs are involved in various biological processes, including cell signaling, inflammation, and tissue repair. They are found in connective tissues, cartilage, skin, and the extracellular matrix of various organs. Some common types of GAGs include hyaluronic acid, chondroitin sulfate, heparan sulfate, keratan sulfate, and dermatan sulfate.
1. Hyaluronic Acid:
- Presence: Found in bacteria and the extracellular matrix of nearly all animals.
- Function: Essential for allowing cells to move during development and healing.
- Bonding: Binds to a protein through the Xylose-Serine O Glycosidic bond.
- Cartilage:. key component of cartilage.
- Bone: Located at areas of calcification in endochondral bone.
2. Keratan Sulfate I and II:
- Keratan Sulfate I:
- First extracted from the cornea.
- Found between collagen fibers in the eye, vital for corneal clarity.
- Keratan Sulfate II:
- Obtained from cartilage.
- Composition: Composed of glucosamine and one of two types of uronic acids.
- Uronic Acids: Iduronic acid may be a notable residue. Initially, all uronic acids are glucuronic acid, but 90% of GlcUA is changed to IdUA by a 5’ epimerase.
3. Heparin:
- Acts as an anticoagulant.
- Binds to lipoprotein lipase in capillary walls, causing its release into the bloodstream.
- Present in mast cell granules, as well as in the liver, lungs, and skin.
Heparan Sulfate
- Heparan sulfate is a type of glycosaminoglycan (GAG) found on many cell surfaces as a proteoglycan. It is different from heparin because the main type of uronic acid in heparan sulfate is glucuronic acid (GlcUA).
- Heparan sulfate acts as a receptor and plays a crucial role in cell growth and cell-to-cell communication.
- It is widely distributed in the body and is the main GAG found in the skin. In the kidney, heparan sulfate is located in the basement membrane, where it is essential for the charge selectivity of glomerular filtration.
- Heparan sulfate is usually found in the extracellular matrix (ECM) and is involved in various physiological processes.
Points to Ponder—GAGs
- GAG without uronic acid: Keratan Sulfate.
- GAG without sulfate group: Hyaluronic Acid.
- GAG not linked to protein: Hyaluronic Acid.
- GAG found in bacteria: Hyaluronic Acid.
- GAG acting as an anticoagulant: Heparin.
- Most abundant GAG: Chondroitin Sulfate.
- GAGs are made in the Endoplasmic Reticulum and Golgi.
- Proteoglycan monomer shape resembles a bottle brush.
- Glycosaminoglycans are polyanions.
- GAG aiding in cell migration under certain conditions: Hyaluronic Acid.
- GAGs aiding cartilage compressibility in weight-bearing: Hyaluronic Acid and Chondroitin Sulfate.
- GAGs contributing to corneal transparency:Keratan Sulfate I and Dermatan Sulfate.
- GAGs determining charge selectivity of renal glomerular membrane: Heparan Sulfate.
- GAGs are mostly found outside cells.
- Some intracellular GAGs: Heparin in mast cells, Heparan sulfate in synaptic and other vesicles.
Mucin Clot Test (Rope Test)
The Mucin Clot Test, also known as the Rope Test, is designed to detect the presence of hyaluronate in synovial fluid.
- In this test, when acetic acid is added to normal synovial fluid, it forms a strong, ropy clot.
Proteoglycan
- Glycosaminoglycans (GAGs) are components that are linked to a protein called the core protein to form molecules known as proteoglycans.
- The link between the polysaccharide chain and the core protein is made through a core trisaccharide: Gal-Gal-Xyl.
- GAGs attach to this core trisaccharide, which is then connected to the core protein.
- However, in the case of hyaluronic acid, GAGs are not linked to a core protein.
- GAGs contribute to the buildup of GAGs in the intralysosomal NBE.
Proteoglycan Monomer and Proteoglycan Aggregate
- Proteoglycan monomers are formed when proteoglycan molecules attach to a core protein.
- These monomers have a bottle brush-like shape.
- Multiple proteoglycan monomers can combine with hyaluronic acid through a link protein, resulting in a proteoglycan aggregate.
- This aggregate is composed of approximately 95% carbohydrates, consisting of long, linear, and unbranched chains, along with short, highly branched oligosaccharide chains.
- The structure features disaccharide repeats, but there are no repeating units within it.
GAG and Diseases
- Tumour cell migration is affected by glycosaminoglycans (GAGs).
- Tumour cells promote the production of hyaluronic acid by fibroblasts.
- Hyaluronic acid facilitates the movement of tumour cells through the extracellular matrix (ECM).
- Some tumour cells have lower levels of heparan sulfate, which may decrease their adhesiveness.
GAG and Atherosclerosis
- Dermatan sulfate is the primary GAG produced by arterial smooth muscle cells.
- These cells proliferate in atherosclerotic lesions within the arteries.
- Dermatan sulfate binds to low-density lipoproteins in the plasma.
- This binding may play a crucial role in the development of atherosclerotic plaques.
GAG and Osteoarthritis
- In cases of arthritis, proteoglycans might act as autoantigens.
- With aging, the level of chondroitin sulfate in cartilage decreases, while the levels of keratan sulfate and hyaluronic acid increase.
- These alterations could contribute to the onset of osteoarthritis.
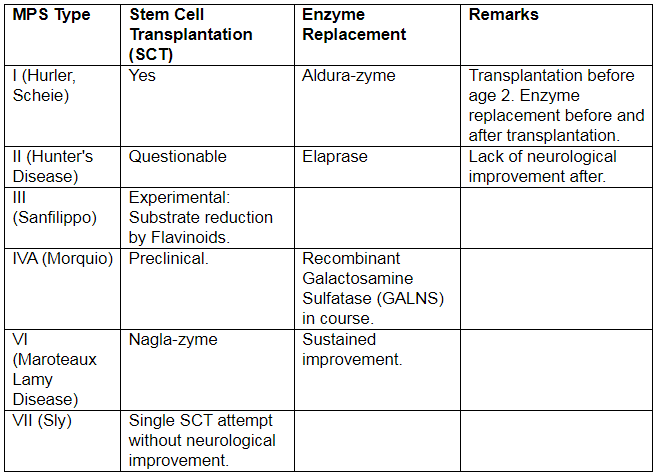
Mucopolysaccharidoses (MPS)
Mucopolysaccharidosis (MPS) is a progressive hereditary disorder caused by mutations in genes responsible for coding lysosomal enzymes essential for degradation.
MPS I (Hurler Disease)
- Inheritance: Autosomal Recessive
- Enzyme Defect: L-Iduronidase
- Urinary Metabolite: Dermatan Sulfate, Heparan Sulfate
MPS I (Scheie Disease)
- Scheie disease
MPS II (Hunter Disease)
- Inheritance: X-linked Recessive
- Enzyme Defect: Iduronate Sulfatase
MPS III A (Sanfilippo A Disease)
- Enzyme Defect: Heparan Sulfate N-sulfatase
MPS III B (Sanfilippo B Disease)
- Enzyme Defect: Glucosaminidase
MPS III C (Sanfilippo C Disease)
- Enzyme Defect: Glucosamine N-acetyl Transferase
MPS III D (Sanfilippo D Disease)
- Enzyme Defect: Glucosamine N-acetyl Transferase
MPS IV A (Morquio A Disease)
- Enzyme Defect: Galactosamine 6-sulfatase
- Urinary Metabolite: Keratan Sulfate, Chondroitin 6 Sulfate
MPS IV B (Morquio B Disease)
- Enzyme Defect: Beta-Galactosidase
MPS VI (Maroteaux-Lamy Syndrome)
- Enzyme Defect: N-Acetyl Galactosamine 4-sulfatase (Aryl Sulfatase B)
MPS VII (Sly Disease)
- Enzyme Defect: Beta-glucuronidase
Note: The important MPS are highlighted in bold letters.
Recognition Pattern of Mucopolysaccharidosis Q2013-14
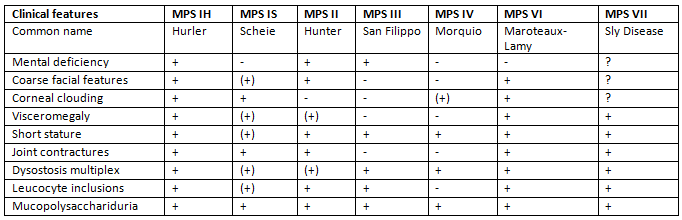
Some Important Mucopolysaccharidoses
Mucopolysaccharidosis-I H (Hurler’s Disease)
Biochemical defect
- Mutations in the IDUA gene on chromosome 4p cause Hurler’s Disease. This gene is responsible for producing α-L-Iduronidase, an enzyme crucial for breaking down certain sugars in the body.
Clinical features of MPS I H (Hurler’s Disease)
- MPS I H is a progressive disorder that impacts multiple organs and tissues, often leading to early death, typically by the age of 10 years .
- Infants with Hurler’s syndrome usually appear normal at birth, though inguinal hernias are commonly seen.
- The diagnosis of this condition is usually made between 6 and 24 months of age.
- Common symptoms include hepatosplenomegaly (enlarged liver and spleen), coarse facial features, corneal clouding, a large tongue, a prominent forehead, joint stiffness, short stature, and skeletal dysplasia.
- Some infants under one year of age may experience acute cardiomyopathy .
- Patients often suffer from recurrent upper respiratory tract and ear infections, noisy breathing, and persistent nasal discharge.
- Valvular heart disease, particularly affecting the mitral and aortic valves, along with narrowing of the coronary arteries, is a common complication.
- Obstructive airway disease, especially during sleep, may necessitate a tracheotomy.
- Common causes of death in these patients include respiratory infections and cardiac issues.
Scheie Disease (MPS-I-S)
- Missense mutations in the IDUA gene on chromosome 4p, which produces α-L-Iduronidase, usually maintain some enzyme activity related to a milder version of the disease.
- Scheie disease shares similarities with MPS I-H in its clinical features.
- MPS I-S is a relatively mild condition characterised by:
- Joint stiffness
- Aortic valve disease
- Corneal clouding
- Mild dysostosis multiplex
- The onset of noticeable symptoms generally occurs after the age of 5 years, with diagnosis usually made between ages 10 and 20.
- Individuals with Scheie disease typically have normal intelligence and height but face significant joint and eye issues.
Mucopolysaccharidosis II (Hunter Disease)
Hunter Disease, also known as Mucopolysaccharidosis II, is an X-linked genetic disorder caused by a deficiency of the enzyme iduronate-2-sulfatase (IDS). This enzyme is crucial for the breakdown of certain complex sugars in the body.
- Studies have shown that approximately 80% of individuals with MPS II have point mutations in the IDS gene, which is located on the X chromosome at Xq28.
- Hunter disease primarily affects males, with only a small number of cases reported in females. This is because females with one normal copy of the IDS gene on their X chromosome can compensate for the defective one, due to the uneven inactivation of the X chromosome.
- The symptoms of MPS II are similar to those of Hurler disease, but there are key differences. Unlike Hurler disease, Hunter disease does not involve corneal clouding and tends to progress more slowly in terms of physical and central nervous system (CNS) decline.
Symptoms:
- Coarse facial features, short stature, joint stiffness, and intellectual disability typically emerge between the ages of 2 and 4 years.
- Some patients may also develop grouped skin papules.
Natowicz Syndrome (MPS-IX)
- Natowicz syndrome, also known as MPS-IX, is caused by a genetic defect in the enzyme hyaluronidase. This condition leads to the accumulation of hyaluronic acid in the joints, as it is not properly broken down.
- MPS-IX is a type of lysosomal storage disorder, where the body's ability to process certain substances is impaired due to the malfunctioning of lysosomes, the cell's recycling units.
- The primary symptoms of Natowicz syndrome include:
- Joint Pain: The buildup of hyaluronic acid in the joints can cause significant discomfort and pain.
- Short Stature: Individuals with this condition may exhibit shorter than average height due to growth-related issues.
Laboratory Diagnosis of Mucopolysaccharidoses
- Urinalysis: Increased levels of glycosaminoglycans (GAGs) are detected in urine samples.
- Enzyme Assays: These tests can be performed on white blood cells, fibroblasts, or possibly serum to measure enzyme activity.
- Tissue Biopsies: Biopsied tissue samples are analyzed for GAGs through electrophoresis.
- Genetic Testing: Specific gene tests are employed to identify mutations.
- Prenatal Diagnosis: Amniotic fluid cells or chorionic villus samples can be used for prenatal testing in certain cases.
Points to Ponder—MPS
- Problems with the breakdown of Heparan Sulfate are associated with cognitive decline.
- Difficulties in degrading Dermatan Sulfate (DS), Chondroitin Sulfate (CS), and Keratan Sulfate (KS) are linked to mesenchymal disorders.
- All types of MPS are inherited in an autosomal recessive manner, except for Hunter's Disease, which is X-linked.
- The most prevalent types of MPS are Sanfilippo Syndrome, followed by Hunter's Syndrome and Hurler's Syndrome. Some MPS cases do not exhibit intellectual disability.
- Morquio Disease is characterized by issues related to Keratan Sulfate and Chondroitin Sulfate degradation.
- Maroteaux Lamy Disease is another variant of MPS.
- MPS types that do not involve corneal cloudinginclude:
- Hunter's Disease.
- Sanfilippo Disease.
- MPS types without visceromegalyinclude:
- Hurler Disease, which shares the same enzyme deficiency as other MPS disorders.
Recent Advances in Treatment Modalities for MPS
Derived Sugars
Derived sugars are monosaccharides that either cannot be described by a general formula or possess unique characteristics. These include:
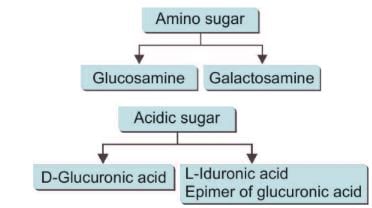
- Acid sugars, which are formed through the oxidation of sugars.
- Sugar alcohols, produced by the reduction of sugars.
- Deoxy sugars, which have one less oxygen atom than the corresponding sugar.
- Amino sugars, where an amino group replaces a hydroxyl group.
- Glycosides, formed when a sugar molecule bonds with another molecule.
- Furfural derivatives, which are compounds derived from furfural.
These derived sugars result from the oxidation of either the aldehyde carbon atom, the hydroxyl carbon atom, or both in monosaccharides.
Effects of Mild Oxidation on Aldehyde Groups
- When the aldehyde group undergoes mild oxidation, it is transformed into Aldonic Acid.
- Glucose is converted into Gluconic Acid, as demonstrated in the Titration of Neutralised Phenolic Group in Extracted Ether 2002.
- Mannose is transformed into Mannonic Acid.
- Galactose changes into Galactonic Acid.
Clinical Application of Glucose to Gluconic Acid
- In the glucose oxidase method for measuring blood glucose levels, gluconic acid is produced from glucose.
- When the aldehyde group of glucose is protected and the last carbon atom is oxidized, uronic acid is formed.
- The conversion of glucose to glucuronic acid involves the formation of iduronic acid, which is an epimer of glucuronic acid and a component of glycosaminoglycans (GAGs).
- Glucuronic acid is also used for the conjugation of bilirubin.
- Additionally, mannose is converted to mannuronic acid, and galactose is converted to galacturonic acid.
Under Strong Oxidation Conditions
- Under strong oxidation conditions, both the first and last carbon atoms of glucose can be oxidized to produce glucosaccharic acid.
- The conversion processes include:
- Glucose to glucosaccharic acid.
- Mannose to mannaric acid.
- Galactose to mucic acid.
Clinical Application: Galactose to Mucic Acid
Mucic acid is the solid crystal utilized in the mucic acid test, which aids in the identification of galactose.
Sugar Alcohols
- Monosaccharides undergo reduction at their carbonyl group, resulting in the formation of related polyhydroxyalcohols.
- Aldoses are reduced to yield their corresponding alcohols.
- Ketoses have the potential to produce two types of alcohols because the reduction process creates a new asymmetric carbon atom.
Sorbitol
- Sorbitol
- Mannitol
- Dulcitol/Galactitol
- Sorbitol and Mannitol
Clinical Uses of Sugar Alcohols
- Mannitol is employed to reduce intracranial pressure by promoting urination.
- The osmotic properties of Dulcitol and Sorbitol can result in cataract formation in Galactosemia and Diabetes, respectively.
- The polyol pathway is associated with the development of diabetic cataracts.
Deoxy Sugars
- In deoxy sugars, a hydroxyl group in the sugar molecule is substituted by a hydrogen atom.
Biochemical Significance of Deoxy Sugars
- Oxygen is removed from the second position in deoxy sugars.
- Deoxy sugars play a vital role in the structure of DNA.
- Feulgen staining is a technique that specifically targets deoxy sugars and DNA in tissue samples.
L-Fucose
- L-Fucose is a type of deoxy sugar that is found in blood group antigens.
2-Deoxy Glucose
- 2-Deoxy Glucose is used in experiments as an inhibitor of glucose metabolism.
Amino Sugars (Hexosamines)
- Amino sugars, also known as hexosamines, are produced when the amino group replaces the hydroxyl group on the second carbon of monosaccharides.
Important amino sugars include:
- Galactosamine (Chondrosamine): Found in various tissues and involved in the formation of cartilage.
- Mannosamine: Plays a role in the synthesis of glycoproteins and glycolipids.
Sialic Acid:
A distinctive amino sugar with nine carbon atoms is sialic acid. The most common form of sialic acid in humans is N-acetyl neuraminic acid (NANA).
Key Considerations in the Metabolism of Amino Sugars
- Glucose as a Precursor: Glucose serves as a precursor for the synthesis of certain amino sugars.
- Precursor of Glucosamine: The immediate precursor of glucosamine is fructose 6 phosphate.
- Source of Amino Group: The amino group required for the synthesis of amino sugars is supplied by glutamine.
- Formation of NANA: N-acetyl neuraminic acid (NANA) is derived from N-acetyl mannosamine.
Biochemical Role of Amino Sugars
- Amino sugars are essential components of glycoproteins, gangliosides, and glycolipids in the body.
- Erythromycin, an antibiotic, contains an amino sugar in its structure.
Glycosides: Definition and Components
- A glycoside forms when a monosaccharide links with an alcohol, phenol, or sterol via an O-glycosidic bond.
- The sugar part is known as the glycone, and the non-sugar part is called the aglycone.
Clinical Significance of Glycosides
- Cardiac Glycosides. These compounds have a significant impact on heart function. Examples include:
- Digitalis. Contains a steroid as the aglycone.
- Quabain. Another cardiac glycoside.
- Antibiotic Glycosides :
- Streptomycin. An antibiotic used to treat various infections.
- Puromycin. Another antibiotic with glycosidic properties.
Isomerism in Carbohydrates
- Isomers are compounds that share the same molecular formula but differ in their structural arrangement.
Asymmetric Carbon Atom
A carbon atom with four different groups attached to it is called a chiral or asymmetric carbon atom.
Le Bel's Rule
Le Bel's Rule describes how the number of asymmetric carbon atoms in a molecule relates to the possible number of stereoisomers.
- The formula for calculating the number of isomers is 2 n .
- In this formula, n represents the number of asymmetric carbon atoms in the molecule.
In the open-chain structure of glucose, there are four asymmetric carbon atoms: C-2, C-3, C-4, and C-5.
However, when glucose is in solution, 99.5% of it is found in the pyranose form. In this form, the first carbon atom does not act as an asymmetric carbon atom. As a result, the total number of possible isomers for glucose is 32.
Different Kinds of Isomers in Carbohydrates
When carbon atoms are asymmetric, it leads to two important characteristics:
- Stereoisomerism
- Optical Isomerism
These are compounds that have the same molecular formula but differ in the way the H and OH groups are arranged around the asymmetric carbon atoms.
D and L Isomerism [Enantiomers]
The arrangement of the H and OH groups around the second-to-last carbon atom creates two mirror images known as D and L isomers ( Enantiomers ).
- The second-to-last carbon atom is referred to as the Reference Carbon.
- In D-glucose, this carbon is C-4.
- Most naturally occurring monosaccharides are D isomers, whereas amino acids are generally L isomers.
Instances of Enantiomers comprise:
- D Glucose and L Glucose
- D Fructose and L Fructose
- D Mannose and L Mannose
- D Glyceraldehyde and L Glyceraldehyde
Anomerism
When monosaccharides form a ring structure, they create an extra asymmetric carbon called the anomeric carbon atom. This carbon atom is where a functional group is attached.
- In glucose, the anomeric carbon is C-1.
- In fructose, C-2 acts as the anomeric carbon.
The arrangement of the H and OH groups around the anomeric carbon leads to the formation of α and β anomers. Examples of anomers include:
- α-D-glucose
- β-D-glucose
Mutarotation
Mutarotation is the change in the optical rotation of plane-polarised light over time, and it is a characteristic of the anomeric carbon atom.
- The optical rotation of α-D-glucose is +112°.
- The optical rotation of β-D-glucose is +19°.
Both forms undergo mutarotation over a few hours, leading to an equilibrium state where the optical rotation is +52°.
Epimerism (a form of diastereoisomerism)
Epimerism refers to a specific type of isomerism that arises from the different arrangements of H and OH groups around carbon atoms in a sugar molecule. This type of isomerism is observed in sugars when the H and OH groups are arranged differently around carbon atoms, except for the anomeric carbon (the carbon that is newly formed during the ring closure of a sugar) and the penultimate carbon (the second-to-last carbon in the chain).
Epimers of Glucose
The variations in the arrangement of H and OH groups at the 2nd, 3rd, and 4th carbon positions (C2, C3, and C4) of glucose lead to the formation of different epimers. These epimers are as follows:
- 2nd epimer of glucose: Mannose
- 3rd epimer of glucose: Allose
- 4th epimer of glucose: Galactose
Optical Isomerism
Optical isomerism in carbohydrates occurs when a beam of plane-polarized light passes through a solution of the carbohydrate, causing the light to rotate either to the right (clockwise) or to the left (anticlockwise). This property gives rise to two types of optical isomers:
- Dextrorotatory isomers (denoted as d or +) rotate the plane of polarized light to the right.
- Levorotatory isomers (denoted as l or -) rotate the plane of polarized light to the left.
For example, D-glucose is a dextrorotatory sugar, which is why it is also called dextrose. In contrast, D-fructose is levorotatory.
A racemic mixture is an equimolar mixture of optical isomers that does not exhibit any rotation of plane-polarized light. An example of this is sucrose, which is also known as invert sugar. Sucrose is dextrorotatory, but when it undergoes hydrolysis, it produces a mixture of dextrorotatory glucose and levorotatory fructose. The strong levorotatory nature of fructose causes the resulting mixture to be levorotatory, leading to the classification of sucrose as invert sugar.
Important Points to Remember about Isomerism
- A monosaccharide without any asymmetric carbon atom is dihydroxyacetone.
- Ketoses have one less asymmetric carbon atom compared to aldoses.
- The number of possible isomers is given by 2 n , where n is the number of asymmetric carbon atoms, according to Leber von’t Hoff rule.
- Not all D isomers are dextrorotatory, and vice versa.
- Glucose and fructose are examples of aldo-keto isomers.
Shapes of Osazones
- Needle-shaped/Broomstick/Sheaves of Corn
- Glucose, Fructose, Mannose
- Pincushion with pins/Hedgehog/Flower of Touch-me-not
- Sunflower Petal-shaped
NB: Sucrose does not form osazones under typical conditions.
Tests for Carbohydrates
- General test for all Carbohydrates.
- Molisch test.
- Test for Reducing Substances.
- Benedict’s Test.
- Test to differentiate Monosaccharides and Disaccharides.
- Barfoed’s Test.
- Moore’s test.
- Fehling’s Test.
- Test to differentiate Aldoses and Ketoses.
- Seliwanoff’s Test.
- Rapid furfural Test.
- Folliger’s Test.
- Test to detect Deoxy Sugar.
- Feulgen Staining.
- Test for Pentoses.
- Bial’s Test.
- Test for Galactose.
- Mucic Acid Test.
Methods for Estimation of Glucose
- Reductometric Methods.
- Nelson Somogyi Method.
- Folin-Wu Method.
- O-Toluidine Method, Enzymatic Method.
- Hexokinase Method.
- Glucose Oxidase Peroxidase Method (GOD–POD ).
- Highly Specific Method.
- This method is used in dry analysis techniques, such as with a Glucometer.
Glucose Oxidase-Peroxidase Method: Reaction Principle
Principle of Glucose Oxidase-Peroxidase Method: Glucose present in the blood sample is oxidized by glucose oxidase to produce gluconic acid and hydrogen peroxide. The hydrogen peroxide then reacts with a chromogen (a substance that can be converted into a dye) in the presence of peroxidase enzyme, leading to a color change. The intensity of this color change is proportional to the amount of glucose in the sample and is measured to determine the glucose concentration.
Dietary Fibers
Dietary Fibers are complex carbohydrates that human digestive enzymes cannot break down. They are also known as non-starch polysaccharides and include:
- Insoluble fibers
- Hemicellulose
- Lignin
- Soluble fibers
- Gums
- Mucilage
The recommended daily allowance is 40 g per 2000 kcal per day, providing 2 kcal/g of energy from dietary fibers.
Beneficial Effects of Dietary Fibers
- Prevent constipation
- Maintain normal motility of the gastrointestinal tract
- Help eliminate bacterial toxins
- Absorb large amounts of water and harmful substances from intestinal bacteria
- Increase the bulk of the stool
- Reduce the time it takes for stool to pass
- Lower the risk of gastrointestinal cancers —such as colon and rectum cancers
- Slow down gastric emptying
- Improve glucose tolerance by slowing glucose absorption
- Lower plasma cholesterol levels
- Decrease the absorption of dietary cholesterol
- Bind bile salts and reduce their circulation, aiding in their excretion
- Provide a sensation of fullness in the stomach
A diet high in fibre is linked to a lower occurrence of:
- Diverticulosis
- Colon cancer
- Cardiovascular disease
- Diabetes mellitus
Dietary fiber is not digested or fermented, such as lignin. In herbivores, colonic bacteria digest dietary fibers, producing small-chain fatty acids like acetate, propionate, and butyrate.
Digestion of Carbohydrates
Carbohydrates are digested by two types of enzymes:
- Alpha amylases
- Disaccharidases
Action of Alpha Amylase
Alpha amylase is an enzyme that plays a crucial role in the digestion of carbohydrates. It is found in various bodily fluids, including:
- Saliva
- Pancreatic juice
- Intestinal juice
When alpha amylase acts on carbohydrates, it breaks them down into smaller components, producing:
- Dextrins, which are intermediate products of starch digestion
- A mixture of glucose, maltose, maltotriose, and small branched dextrins derived from the branch points of amylopectin, a component of starch
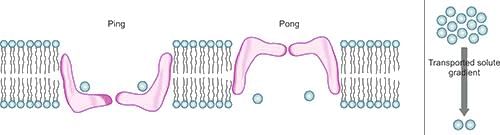
Glucose Transporters
- Glucose transport is a passive process that occurs down the concentration gradient.
- It is bidirectional, meaning glucose can be transported in both directions.
- The process involved is facilitative diffusion, which does not require energy.
- The Ping Pong Mechanism is a specific way glucose is transported.
- This mechanism is sodium-independent, meaning it does not rely on sodium ions for transport.
Action of Disaccharidases
- Maltase, Sucrase-Isomaltase, Lactase, and Trehalase are enzymes that act on disaccharides.
- These enzymes are located on the brush border of intestinal mucosal cells.
- They break down disaccharides into their monosaccharide components, which are then absorbed by the body.
Lactase Deficiency and Sucrase Deficiency
Sucrase Deficiency
- Symptoms may arise after consuming milk products due to lactose.
- Symptoms can also occur after eating dairy products containing sucrose.
- Common symptoms include watery diarrhea, bloating, and failure to thrive.
Absorption of Carbohydrates
- Carbohydrates are absorbed through two types of transporters:
- Sodium-dependent glucose transporters (SGLT)
- Sodium-independent glucose transporters (GLUTs)
Sodium-dependent glucose transporters
Secondary active transport
- Unidirectional
- SGLT is linked with the Na+/K+ ATPase pump.
- Sodium-dependent Glucose Transporters (SGLT)
SGLT1
- Location: Small intestine, renal tubules
- Function: Absorption of glucose
SGLT2
- Clinical Correlation—Renal Glycosuria
- Isolated glucosuria occurs with normal blood glucose due to mutations in SLC5A2.
- SLC5A2 encodes the high-capacity sodium-glucose co-transporter SGLT2 in the proximal renal tubule.
Tissue Location
GLUT 1
- Location: Brain, kidney, colon, placenta, RBCs, retina
- Function: Basal glucose uptake
GLUT 2
- Location: Liver sinusoid membrane, β cells of pancreas, basolateral side of intestinal cell, PCT
- Function: Removes excess glucose from blood in liver; regulates insulin release in pancreas.
- Characteristics: Low affinity and higher Km.
GLUT 3
- Location: Neurons, placenta
- Characteristics: High affinity for glucose
GLUT 4
- Location: Heart, skeletal muscle, adipose tissue
- Characteristics: Insulin-dependent
GLUT 5
- Location: Small intestine, testis, sperm
- Function: Primarily a fructose transporter
GLUT 6
- Location: Spleen, leukocytes
- Characteristics: Possibly no transporter function
GLUT 7
- Location: Liver endoplasmic reticulum
- Function: Glucose transporter in the endoplasmic reticulum
Functions
GLUT 8
- Location: Testis, blastocyst, brain
- Characteristics: Insulin-responsive
- Function: Glucose transporter for mature spermatozoa
GLUT 9
- Location: Liver, kidney
- Function: Urate transporter
GLUT 10
- Location: Liver, pancreas
GLUT 11
- Location: Heart, skeletal muscle
- Function: Fructose transporter
GLUT 12
- Location: Prostate, heart, mammary gland, white adipose tissue
- Characteristics: Insulin responsive
- Other glucose transporters are:
Inhibitors of Glucose Transporters
- Phlorizin (Phloretin 2 β-D-Glucoside) inhibits the sodium-dependent glucose transporter by competing with D-glucose for binding sites. Its effectiveness is greater in SGLT2 compared to SGLT1.
- Phloretin, the aglycone of phlorizin, blocks facilitated diffusion through GLUT-1 or GLUT-4.
Key Considerations Regarding Glucose Transporters
- GLUT-1 is the widely distributed and most abundant glucose transporter in red blood cells.
- GLUT-1 is also the primary glucose transporter in the brain and the principal one in the placenta.
- GLUT-3 is the major glucose transporter in neurons, while GLUT-1 is present in neurons, especially at the blood-brain barrier.
- GLUT-8 is the glucose transporter in the blastocyst and is also insulin-dependent, along with GLUT-4 and GLUT-12.
- GLUT-9 is the urate transporter.
Absorption of Monosaccharides
- Glucose and galactose are absorbed through a sodium-dependent process using the same transport protein, SGLT-1, and compete for absorption in the intestines.
- Fructose is absorbed down its concentration gradient via GLUT-5.
- All sugars exit from intestinal cells using GLUT-2.
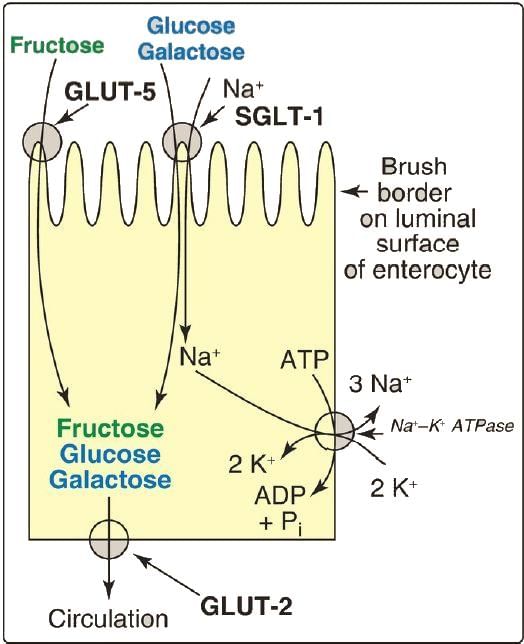 Absorption of monosaccharides
Absorption of monosaccharides
|
48 docs|7 tests
|
FAQs on Chemistry of Carbohydrates Chapter Notes - Biochemistry - NEET PG
| 1. What is the general formula for carbohydrates? |  |
| 2. What are monosaccharides and can you provide an example? |  |
| 3. What distinguishes reducing disaccharides from nonreducing disaccharides? |  |
| 4. What are homoglycans and how do they differ from heteroglycans? |  |
| 5. What are mucopolysaccharides and why are they biologically significant? |  |




















