Inflammation Chapter Notes | Pathology - NEET PG PDF Download
Introduction
Inflammation is the body's response to injury, involving blood vessels and cells. It can be:
- Acute inflammation: Short-term response lasting from seconds to a few hours.
- Chronic inflammation: Prolonged response lasting weeks, months, or even years.
Inflammation triggers changes in blood vessels (vascular changes) and cells (cellular changes).
I. Vascular Changes
- Vasoconstriction: Initial, temporary response causing blood vessels to constrict, making the skin pale after an injury.
- Vasodilation: Subsequent response that lasts longer, increasing blood flow, leading to redness (rubor) and warmth (color).
- Increased permeability: Characteristic of acute inflammation, caused by the separation of endothelial cells. This allows fluid, cells, and proteins to exit blood vessels, resulting in exudate. This protein-rich fluid causes swelling (tumor) at the injury site, particularly in venules.
Mechanisms of Increased Vascular Permeability
- Formation of endothelial gaps (Immediate transient response): Endothelial gaps form due to vasoactive mediators like histamine and contraction of endothelial cells. This mechanism affects venules and is rapid and reversible, lasting 15 to 30 minutes.
- Direct endothelial injury (Immediate sustained response): Caused by toxins or infections, leading to damage in venules, capillaries, and arterioles. This response is fast but can be long-lasting.
- Cytoskeletal reorganization: Triggered by cytokines and hypoxia, mainly affecting venules with some impact on capillaries. This mechanism is reversible but delayed and prolonged.
- Delayed prolonged leakage: Resulting from thermal and radiation damage, affecting venules and capillaries. This response is delayed and long-lasting.
- Leukocyte-mediated endothelial injury: Caused by activated leukocytes, primarily affecting venules. This mechanism is late and long-lasting.
- Increased transcytosis: Involves the formation of vesicles near cell junctions by histamine and vascular endothelial growth factor (VEGF).
- Leakage from new blood vessels: Influenced by vascular endothelial growth factor (VEGF), occurring at sites of angiogenesis.
Fluid loss during inflammation leads to the concentration of red blood cells in small vessels, increasing blood viscosity, a condition known as stasis. Increased vascular permeability is a hallmark of acute inflammation, with the formation of endothelial gaps being the most common mechanism.
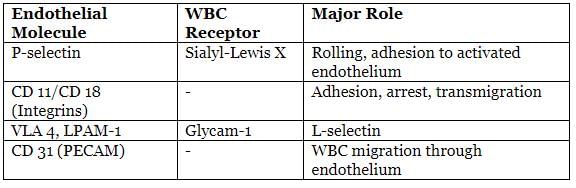
Selectins are crucial in the "rolling" of neutrophils along the endothelium, with E-selectin playing a significant role in acute inflammation. β2-Integrins are adhesion molecules that facilitate the attachment of neutrophils to blood vessels.
- LAD1 (Leukocyte Adhesion Deficiency Type 1): Integrin defects lead to recurrent infections and delayed separation of the umbilical cord stump.
- LAD2 (Leukocyte Adhesion Deficiency Type 2): Selectin defects cause recurrent infections, the Bombay blood group, and developmental delays.
II. Cellular Changes
The movement of leukocytes from blood vessels to tissues, known as extravasation, involves several steps:
- Margination: Leukocytes move from the center of the blood vessel to the edges.
- Rolling:Temporary adhesion of leukocytes to endothelial cells, primarily mediated by selectins. Types of selectins include:
- E-selectin (CD62E): Found on activated endothelial cells; interacts with sialyl Lewis X on leukocytes.
- P-selectin (CD62P): Present on platelets and endothelial cells; interacts similarly with leukocytes.
- L-selectin (CD62L): Found on leukocytes; interacts with various adhesion molecules on endothelial cells.
- Adhesion:Firm attachment of leukocytes to endothelial cells, primarily mediated by integrins. These can be:
- β1-containing integrins. Also known as VLA molecules, interact with VCAM-1 on endothelial cells.
Clinical Relevance of Adhesion Molecules
β2-Integrins (LFA-1 and Mac-1)
These molecules play a crucial role in the interaction between leukocytes and endothelial cells during the immune response.
Types of Leukocyte Adhesion Deficiency (LAD)
1. Leukocyte Adhesion Deficiency Type 1 (LAD1)
- Cause: Defect in the CD18 molecule, which is essential for forming the β2 chain in LFA-1 and Mac-1 integrins.
- Consequence: Poor adhesion resulting in defective aggregation and impaired chemotaxis.
- Symptoms: Recurrent bacterial infections (skin, oral, genital, respiratory, and intestinal), persistent leukocytosis, and delayed separation of the umbilical cord stump.
2. Leukocyte Adhesion Deficiency Type 2 (LAD2)
- Cause: Absence of sialyl-Lewis X, a ligand for E-selectin, due to a defect in the enzyme fucosyl transferase.
- Symptoms: Recurrent bacterial infections, platelet dysfunction, short stature, association with the Bombay blood group, and mental retardation.
- Classification: Known as “congenital disorder of glycosylation IIc.”
3. Leukocyte Adhesion Deficiency Type 3 (LAD3)
- Cause: Mutation in the FERMT3 gene affecting integrin activation signaling.
- Symptoms: Petechial hemorrhage, leukocytosis, and recurrent infections.
Note: LAD1 and LAD2 are inherited in an autosomal recessive manner.
Endothelial Leukocyte Adhesion Molecules and Their Functions
Transmigration
Transmigration, also known as diapedesis, is the process through which leukocytes move through the endothelium to reach the site of infection or inflammation. The key molecule involved in this process is PECAM-1 (platelet endothelial cell adhesion molecule) or CD31. During an inflammatory response, neutrophils are the first cells to arrive at the site, typically within the first 6 to 24 hours. They are followed by monocytes, which arrive within 24 to 48 hours, except in cases of Pseudomonas infections where neutrophils may remain dominant for 2 to 4 days.
Chemotaxis
Chemotaxis is the process by which leukocytes move in a unidirectional manner towards antigens or bacteria in response to specific chemicals known as chemotactic stimuli. These stimuli can be exogenous, such as bacterial products, or endogenous, such as C5a, LTB4, and IL-8. All chemotactic agents bind to G-protein coupled receptors (GPCRs) on the surface of leukocytes, triggering actin polymerization and subsequent movement. Other proteins, including filamin, gelsolin, profilin, and calmodulin, also play a role in facilitating contraction and movement by interacting with actin and myosin. Upon activation, leukocytes degranulate, releasing lysosomal enzymes, cytokines, and producing arachidonic acid metabolites. This activation can occur through GPCRs, cytokine receptors, and Toll-like receptors (TLRs).
Opsonisation
Opsonisation is the process of coating bacteria to enhance their recognition and ingestion by white blood cells (WBCs). This process makes bacteria more appealing to leukocytes, similar to how water enhances the flavor of golgappa, a popular snack. While WBCs can kill bacteria without opsonins, opsonised bacteria are preferentially eliminated.
Opsonins are substances that promote opsonisation and include:
- C3b
- Fc fragment of antibodies or IgG
- Serum proteins such as fibrinogen, mannose-binding lectin, and C-reactive protein
Phagocytosis
Phagocytosis is the process through which bacteria are engulfed and destroyed by white blood cells. Lysosomes are essential organelles required for phagocytosis.
Steps of Phagocytosis
- Recognition and attachment:Particles to be ingested, such as microbes and dead cells, are recognized by receptors on WBCs. These receptors include:
- Scavenger receptors: Bind microbes and oxidized or acetylated LDL particles.
- Mac-1 integrins: Found on the surface of macrophages.
- Mannose receptors: Bind to mannose and fucose residues of glycoproteins in microbial cell walls.
- The presence of an extra terminal sialic acid or N-acetyl galactosamine in human cells protects them from WBC destruction.
- Engulfment: Formation of a phagolysosome occurs when lysosomes fuse with the phagosome containing the microbe, followed by degranulation of leukocytes.
Clinical Importance of Phagolysosome Formation
Phagolysosome formation is clinically significant in a genetic disorder known as Chediak-Higashi syndrome. This syndrome is characterized by:
- Decreased transfer of lysosomal enzymes to phagocytic vacuoles.
- Impaired degranulation.
- Delayed microbial killing, leading to an increased susceptibility to infections.
- Polymorphonuclear cells (polymorphs) exhibiting poor random movement and defective chemotaxis.
Clinical Features of Chediak-Higashi Syndrome
- Neutropenia:. low count of neutrophils in the blood.
- Albinism:. condition caused by defects in melanocytes, leading to reduced pigmentation.
- Nerve Defects: Abnormalities in the nerves.
- Nystagmus: Involuntary eye movements.
- Bleeding Disorders: Resulting from defects in neurons and platelets.
- Reduced NK Cell Responsiveness: Decreased activity of natural killer cells.
The secretion of granule proteins by cytotoxic T cells is also impaired, contributing to immunodeficiency. The gene responsible for lysosomal trafficking in this syndrome is called LYST.
Leukocyte Diapedesis
Leukocyte diapedesis, similar to increased vascular permeability, primarily occurs in venules and capillaries. However, in the lungs, it also takes place in capillaries.
Examples of Opsonins
- Antibodies: Proteins produced by B cells that bind to specific antigens, marking them for destruction.
- Complement Proteins: Part of the immune system that enhances the ability of antibodies and phagocytic cells to clear pathogens.
- Lectins: Proteins that bind to carbohydrates on the surface of pathogens, facilitating their recognition and clearance.
Chemotaxis
- Chemotaxis is the process by which leukocytes move in a directed manner towards antigens or bacteria.
IgG Production and Bruton’s Disease
- IgG antibodies are produced by activated B cells known as plasma cells. In a condition called Bruton’s disease, B cell maturation is impaired, leading to the absence of immunoglobulins. This deficiency results in defective opsonization, where pathogens are marked for destruction by antibodies.
Mechanisms of Endocytosis
- Phagocytosis: Involves the polymerization of actin filaments to engulf large particles.
- Pinocytosis and Receptor-Mediated Endocytosis: Rely on clathrin-coated pits to internalize fluids and specific molecules.
Chediak-Higashi Syndrome and Giant Granules
- In Chediak-Higashi syndrome, leukocytes contain giant granules that are visible in peripheral blood smears. These granules result from the abnormal fusion of organelles within the cells.
O2-Dependent MPO System
- The O2-dependent myeloperoxidase (MPO) system is considered the most effective microbicidal mechanism for killing bacteria.
NADPH Oxidase and Respiratory Burst
- NADPH oxidase, also known as the respiratory burst oxidase, plays a crucial role in generating reactive oxygen species (ROS) during the respiratory burst. This process is vital for the killing of ingested bacteria.
Chronic Granulomatous Disease (CGD)
- Definition: Chronic granulomatous disease is a genetic disorder characterized by a defect in the NADPH oxidase enzyme, leading to impaired respiratory burst and the inability to produce reactive oxygen species for microbial killing.
- Symptoms: Individuals with CGD experience recurrent infections due to the inability to kill certain bacteria and fungi. Common infections are caused by catalase-positive organisms such as Staphylococcus aureus and fungi like Candida.
- Diagnosis: CGD can be diagnosed using the Nitroblue-tetrazolium (NBT) test, which measures the ability of phagocytes to produce superoxide radicals. In healthy individuals, NBT is reduced to formazan by superoxide, indicating normal NADPH oxidase activity. In CGD, this reaction does not occur, resulting in a negative test.
- Dihydrorhodamine (DHR) Test: An advanced test that assesses the ability of phagocytes to produce superoxide by measuring the reduction of DHR to rhodamine in cells with normal function.
Difference between Myeloperoxidase Deficiency and Chronic Granulomatous Disease (CGD)
- In CGD: Ingested organisms, such as catalase-negative bacteria like Streptococci, can be killed because these organisms produce their own hydrogen peroxide. This hydrogen peroxide is utilized by neutrophilic myeloperoxidase to create free radicals that eliminate the bacteria.
- In myeloperoxidase deficiency: The enzyme myeloperoxidase is absent. As a result, both catalase-positive and catalase-negative organisms survive within phagocytes, leading to recurrent infections.
Enzymes Involved in Respiratory Burst
The respiratory burst in phagocytes involves several key enzymes that play a crucial role in generating reactive oxygen species for microbial killing. These enzymes include:
- NADPH Oxidase: This enzyme is responsible for the initial step of the respiratory burst, where molecular oxygen is reduced to superoxide free radicals. NADPH oxidase is a multi-subunit enzyme complex found in the membranes of phagocytes.
- Superoxide Dismutase (SOD): SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide and oxygen, facilitating the conversion of harmful superoxide into less toxic forms.
- Myeloperoxidase (MPO): MPO is an enzyme found in the azurophilic granules of neutrophils. It uses hydrogen peroxide, produced during the respiratory burst, to generate hypochlorous acid (HOCl) from chloride ions. HOCl is a potent antimicrobial agent.
- Catalase: While not directly involved in the generation of reactive oxygen species, catalase plays a protective role by decomposing hydrogen peroxide into water and oxygen, preventing oxidative damage to host tissues.
Role of Reactive Oxygen Species in Phagocytosis
Reactive oxygen species generated during the respiratory burst play a vital role in the final stages of phagocytosis by killing and degrading ingested microbes. This process involves various mechanisms, including the production of harmful oxidative species that damage bacterial components, leading to their destruction and clearance from the host.
NADPH oxidase
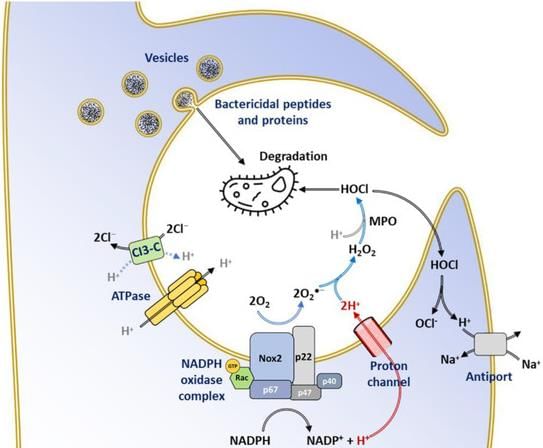
NADPH oxidase is the main enzyme responsible for producing hydrogen peroxide (H2O2), which is crucial for killing microbes.
- Catalase is an enzyme that breaks down hydrogen peroxide into water and oxygen.
- Superoxide dismutase (SOD) is an enzyme that converts superoxide ions into hydrogen peroxide.
- Glutathione peroxidase is an enzyme that converts reduced glutathione to its homodimer.
Note: The H2O2 – MPO – halide system is the most effective method for killing bacteria.
Oxygen-Independent Killing Mechanism
The oxygen-independent killing mechanism can occur through various enzymes and proteins, such as:
- Lysozyme: Breaks down the glycopeptide coat of bacteria.
- Lactoferrin: An iron-binding protein that inhibits bacterial growth by depriving them of iron.
- Bacterial permeability increasing protein: Increases the permeability of bacterial membranes, leading to their death.
- Major basic protein (MBP):. protein that is toxic to parasites and involved in the immune response.
- Defensins: Peptides rich in arginine that have antimicrobial properties and are toxic to microbes.
- Cathelicidins: Antimicrobial proteins found in neutrophils and other cells, which are highly effective against M. tuberculosis and other pathogens.
Chemical mediators of inflammation can exist in either cellular form or plasma.
- Major basic protein is found in eosinophils and is toxic to parasites.
- The synthesis of the antimicrobial protein cathelicidin is stimulated by 1,25 dihydroxyvitamin D. This non-skeletal effect is important because a deficiency in vitamin D can raise the risk of tubercular infections.
Histamine is produced from the amino acid ‘histidine.’ Mast cells are the richest source of histamine. It is also found in platelets and basophils, causing:
- Vasodilation (but vasoconstriction in large arteries).
- Increased permeability (immediate transient response).
- Bronchoconstriction.
Serotonin (5-HT) is similar to histamine and is found in platelets and enterochromaffin cells.
- Lysosomal enzymes are found in the lysosomes of neutrophils and monocytes and include primary (azurophilic) and secondary (specific) granules.
Histamine is the main chemical mediator of acute inflammation.
- Two major anti-proteases are α1 antitrypsin and α2 macroglobulin.
- Neutrophils have tertiary granules, known as C particles, containing gelatinase and acid hydrolases.
Newly Synthesized Cellular Mediators
Nitric Oxide: Nitric oxide is synthesized from the amino acid L-arginine by the enzyme nitric oxide synthase (NOS). There are three isoforms of NOS in the body:
- eNOS (endothelial nitric oxide synthase) is found in the endothelium and is constitutively expressed.
- nNOS (neuronal nitric oxide synthase) is found in neurons and is also constitutively expressed.
- iNOS (inducible nitric oxide synthase) is induced by cytokines such as TNFα (tumor necrosis factor-alpha) and IFN-γ (interferon-gamma).
Important Actions of Nitric Oxide: Nitric oxide has several important actions in the body, including:
- Strong vasodilator: Nitric oxide relaxes blood vessels, leading to increased blood flow.
- Reduces platelet aggregation: Nitric oxide inhibits the aggregation of platelets, reducing the risk of blood clots.
- Regulates leukocyte recruitment: Nitric oxide plays a role in the recruitment of leukocytes (white blood cells) to sites of inflammation.
- Microbicidal effects: Nitric oxide acts as a free radical and can form highly reactive compounds such as peroxynitrite (ONOO–), nitrogen dioxide (NO2), and nitrate (NO3), which have microbicidal effects.
Cytokines: Cytokines are small protein molecules secreted by inflammatory cells, including interleukins, interferons, and tumor necrosis factor-alpha (TNF-α). They can have local and systemic effects. IL-1 and TNF-α are crucial for systemic inflammatory responses.
- IL-1 and TNF-α are involved in the acute phase reaction, affecting endothelial and fibroblast formation.
- IL-1, IL-6, and TNF-α increase procoagulant activity and promote fibroblast and collagen formation.
- Interferons contribute to the acute phase response.
Effects on White Blood Cells (WBCs): Cytokines increase the secretion of IL-1 and IL-6 by white blood cells, further amplifying the inflammatory response.
Overview of Chemical Mediators in Inflammatory Response
- Resolvins and protectins are substances that help reduce inflammation. They are made from polyunsaturated fatty acids and work together with other compounds like IL-10, TGF-β, and lipoxins to prevent acute inflammation.
- Arachidonic acid (AA) is a fatty acid with 20 carbon atoms and four double bonds. To convert AA into other compounds, it must be freed from membrane phospholipids.
- COX Pathway. There are two types of COX enzymes, COX-1 and COX-2. These enzymes convert AA into PGG2 and then into PGH2. The final product of PGH2 depends on the specific enzyme present in a cell. For example, in the endothelium, PGH2 is converted into PGI2, while in platelets, it is converted into TXA2.
- FLAP stands for Five Lipoxygenase Activating Protein, which plays a role in the LOX pathway.
LOX Pathway. Arachidonic acid can be processed by various types of LOX enzymes.
- 5-LOX is found in leukocytes, mast cells, and dendritic cells. It works with FLAP to convert AA into LTA4, which can then be transformed into either LTB4 or cysteinyl leukotrienes (LTC4, D4, E4).
- 15-LOX converts AA into 15-HETE, which can be further converted into lipoxins (LXA4 and LXB4) with the help of 5-LOX. Lipoxins can also be produced by 12-LOX acting on LTA4.
Functions of Key Compounds
- LTB4 is a potent attractant for T-lymphocytes, eosinophils, monocytes, and mast cells.
- LTC4, D4, and E4 attract T-lymphocytes and eosinophils.
- Lipoxins activate monocytes and macrophages while inhibiting neutrophils. They also reduce NK cell activity and act as strong coronary vasoconstrictors in living organisms.
Epoxygenase Pathway. Cytochrome P450 can convert AA into 20-HETE or EET. The biological effects of EET are reduced when it is converted to the less active DHET by epoxide hydrolases.
- EET may act as an endothelium-derived hyperpolarising factor, particularly in coronary blood flow. It also has anti-inflammatory, anti-apoptotic, and pro-angiogenic effects.
- 20-HETE can cause constriction of blood vessels in the kidneys and is associated with high blood pressure. However, EET has properties that lower blood pressure through vasodilation and promoting sodium excretion. Drugs that inhibit epoxide hydrolase, thereby increasing EET levels, are being developed for the treatment of hypertension.
COX Enzymes
- COX-1 is typically always present in cells (housekeeping function), while COX-2 is usually induced in response to specific stimuli. However, both COX-1 and COX-2 are present in the endothelium, kidneys, and central nervous system (CNS).
Prostaglandins
- PGF2α is involved in blood vessel constriction.
- PGD2 and PGE2 are associated with blood vessel dilation.
- PGI2 plays a role in preventing platelet aggregation (the "i" stands for inhibition), while TXA2 promotes platelet aggregation (the "a" stands for aggregation).
Leukotrienes
- LTC4 and LTD4 are key components of the slow-reacting substance of anaphylaxis. They are strong bronchoconstrictors and increase permeability and mucus production in the airways.
Chemokines
- IL-8 is a chemokine that attracts neutrophils, while Eotaxin specifically attracts eosinophils.
- Chemokines are small proteins that mainly attract specific types of white blood cells. They are classified into four main groups based on the arrangement of their conserved cysteine residues. Most chemokines have four conserved cysteines, indicated by C. X represents any amino acid other than cysteine. For example, C-X-C means two cysteines are separated by one amino acid, and C-X3-C means they are separated by three. C-C indicates no separation, while C-chemokines lack the first and third conserved cysteines.
- Chemokines exert their functions through chemokine receptors, such as CXCR or CCR. Some receptors, like CXCR4 and CCR5, facilitate the binding of HIV to CD4 cells.
Complement System Activation
- Activating C3, the most common component, is a crucial step in the functioning of the complement system.
Abbreviations
- DHET. Dihydroxyeicosatrienoic acid
- EET. Epoxyeicosatrienoic acid
- HETE. Hydroxyeicosatetraenoic acid
- HPETE. Hydroxyperoxyeicosatetraenoic acid
Isoeicosanoid Pathway. Isoprostanes are prostaglandin stereoisomers formed from the non-enzymatic free-radical oxidation of AA and similar lipids. They have strong vasoconstrictive effects and influence interactions between white blood cells and platelets, as well as blood vessel growth.
Mediators Present in Plasma
Complement System
- The complement system is made up of about 20 proteins, known as complement proteins, which are found in the plasma. These proteins are numbered from C1 to C9, including their breakdown products.
- There are four main pathways in the complement system:
- Classic Activation Pathway. This pathway is triggered by antigen/antibody immune complexes.
- Mannose Binding Lectin Activation Pathway. This pathway is activated by microbes that have terminal mannose groups on their surface.
- Alternative Activation Pathway. This pathway is activated by the presence of microbes or tumor cells.
- Terminal Pathway. This pathway is common to the first three and leads to the formation of the Membrane Attack Complex (MAC), which lyses (destroys) cells.
- All pathways ultimately result in the breakdown of a protein called C3, leading to the formation of the MAC, which is crucial for destroying antigens.
- Classical Pathway Activation. When this pathway is activated, there is a decrease in the levels of complement proteins C1, C2, C4, and C3, while factor B remains normal.
- Alternative Pathway Activation. This activation shows decreased levels of factor B and C3, with normal levels of C1, C2, and C4.
- The C1 inhibitor (C1 INH) is a regulatory protein that prevents C1 from binding to immune complexes. When C1 INH is deficient, it leads to excessive complement activation, causing a condition known as hereditary angioneurotic edema.
- Functions of Important Individual Complement Proteins
- C3a and C5a. These proteins are called anaphylatoxins because they trigger the release of histamine from mast cells. This process leads to vasodilation (widening of blood vessels) and increased vascular permeability (leakiness of blood vessels).
- C3b and inactive C3 (C3i). These proteins are important for opsonization, which is a process that marks pathogens for destruction by immune cells.
- C5a. This protein plays a crucial role in chemotaxis, which is the movement of immune cells towards the site of infection or inflammation.
- C5b-9 (MAC). This complex is responsible for attacking and killing pathogens by forming pores in their cell membranes.
- Regulatory Molecules of the Complement System
- Decay Accelerating Factor (DAF). This factor promotes the dissociation of C3 convertase, an enzyme complex that plays a key role in the complement activation process.
- Factor I. This factor is involved in cleaving C3b, a complement protein, thus regulating the complement activation.
- CD59. This protein inhibits the formation of the Membrane Attack Complex (MAC), thereby preventing cell lysis.
- Factor H, Factor I, and CD46. These molecules work together to prevent excessive activation of the alternative pathway of the complement system, ensuring that the complement response is balanced and controlled.
- Deficiency of Complement Component
- C1 Esterase Inhibitor. Its deficiency leads to Hereditary angioneurotic edema, characterized by subcutaneous swelling due to excessive complement activation.
- Early Complement Proteins C1, C2, C4. Deficiencies in these proteins are associated with conditions like Systemic Lupus Erythematosus (SLE) and collagen vascular disorders.
- C3 and C3 Inactivator. Deficiencies can result in recurrent pyogenic infections due to impaired opsonization and immune response.
- C5 and C8. Deficiencies increase susceptibility to bacterial infections, particularly with Neisseria species, and Toxoplasmosis.
- C2 Deficiency. This is the most common complement deficiency, linked with increased risk of Streptococcal septicemia and a lupus-like syndrome in children.
- Activation by Antibodies
- IgM and IgG. These antibodies, especially IgM, activate the classical pathway of the complement system.
- IgA. This antibody activates the alternative pathway.
- Clotting Factors Production
- Most clotting factors are produced in the liver.
- Exception. Factor IV (calcium) and von Willebrand factor, which carries factor VIII, are not produced in the liver.
- Vitamin K Requirement
- Factors II (prothrombin), VII, IX, and X require vitamin K for their activation.
- Monitoring Coagulation Pathways
- The intrinsic pathway is monitored using activated partial thromboplastin time (aPTT).
- The extrinsic pathway is monitored using prothrombin time (PT) or International Sensitivity Index (ISI).
- Factor XII Deficiency
- Factor XII deficiency does not lead to bleeding.
- Instead, it results in a prothrombotic state, increasing the risk of thrombosis.
- Fibrin Degradation
- Fibrin is broken down by plasmin into smaller fibrin degradation products (FDPs).
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- DAF and CD59. Deficiencies in these proteins lead to PNH, characterized by complement-mediated increased intravascular lysis of red blood cells (RBCs), platelets, and neutrophils.
- Atypical Hemolytic Uremic Syndrome (HUS)
- CD46, Factors H and I. Deficiencies in these proteins are associated with atypical or ‘non-epidemic’ HUS, a condition characterized by hemolytic anemia, acute renal failure, and thrombocytopenia.
- Clotting System Overview
- The clotting system's primary function is to form a blood clot to prevent excessive blood loss.
- Some components, like fibrinogen, are utilized for opsonization, while thrombin induces chemotaxis.
- Clinical Correlation. Deficiency of clotting factors VIII and IX results in hemophilia A and B, respectively.
- Bleeding in Hemophilia Despite Normal Extrinsic Pathway
- The extrinsic pathway initiates limited thrombin activation upon tissue injury, which is crucial for hemostasis.
- Thrombin further activates factors XI and IX of the intrinsic pathway, reinforcing the coagulation process.
- High levels of thrombin are necessary to activate TAFI, enhancing fibrin deposition by inhibiting fibrinolysis.
- In hemophilia, inadequate coagulation (fibrinogenesis) and inappropriate clot removal (fibrinolysis) contribute to bleeding.
- Kinin System Activation
- The kinin system is activated by factor XII (Hageman’s factor).
- Kallikrein, an enzyme in this system, can activate plasminogen to plasmin and complement protein C5a.
Contraction of Smooth Muscles
- Pain
- Dilation of the venules
The most important outcome of acute inflammation is the clearance of the injurious stimuli and replacement of injured cells (resolution).
Morphological Patterns of Inflammation
- Serous inflammation is marked by the release of a clear fluid.
- Fibrinous inflammation involves the deposition of fibrin in tissues.
- Catarrhal inflammation is characterized by increased production of mucus.
- Purulent inflammation is associated with the formation of pus.
- Effusion refers to the presence of fluid in body cavities.
- Inflammation can lead to the accumulation of fluid in body cavities.
Chronic Inflammation
Chronic inflammation is characterized by the infiltration of mononuclear cells such as macrophages, lymphocytes, and plasma cells. This leads to tissue damage and subsequent repair processes, including the formation of new blood vessels and scarring. The predominant cell type in chronic inflammation is the macrophage, which can accumulate in tissues due to recruitment from the bloodstream, local proliferation, and persistence at the site of inflammation. A hallmark of chronic inflammation is tissue destruction.
Types of Macrophages:
- Kupffer cells (found in the liver)
- Microglia (found in the brain)
- Osteoclasts (found in bone)
- Alveolar macrophages or 'dust cells' (found in the lungs)
- Histiocytes (found in connective tissue)
- Hofbauer cells (found in the placenta)
- Littoral cells (found in the spleen)
- Mesangial cells (found in the kidneys)
- Type A lining cells (found in the synovium)
Activated factor XII can initiate the activation of various systems, including the kinin system, clotting system, fibrinolytic system, and complement system.
- Catarrhal inflammation is the most prevalent form of inflammation.
- An abscess refers to a localized collection of pus-forming inflammatory tissue.
- Infections are the primary triggers of chronic inflammation.
- Monocytes and macrophages play a crucial role as the main white blood cells in chronic inflammation.
- Epithelioid cells are specialized macrophages activated by interferon γ from CD4 T cells.
- Warthin-Finkeldey giant cells are associated with measles infections.
- Tumour giant cells, such as Reed-Sternberg cells, are linked to Hodgkin's lymphoma.
- Among inflammatory bowel diseases (IBD), only Crohn's disease is characterized by the presence of these cells, whereas they are absent in ulcerative colitis.
Subsets of Activated Macrophages
- Classically activated macrophages (M1)
- Alternatively activated macrophages (M2)
- Triggered by microbial products and cytokines like IFN-γ.
- Triggered by microbial products and cytokines like IL-4, IL-5.
- Release lysosomal enzymes, nitric oxide, IL-1, and IL-12.
- Release IL-10 and TGF-β.
- Involved in microbicidal activities and pathogenic inflammation.
- Involved in anti-inflammatory actions and wound repair.
Granulomatous Inflammation
Cells can be grouped based on their ability to regenerate:
- Permanent cells. Cells that do not divide, such as neurons, skeletal muscle fibres, and cardiac myocytes.
- Stable cells. These have a low rate of division and mainly stay in the G0 phase. When stimulated, they enter the G1 phase and multiply, like cells in the proximal tubule of the kidney, hepatocytes, pancreatic cells, and fibroblasts.
- Labile cells. These can regenerate throughout life, such as haematopoietic cells, cells of the skin, and the gastrointestinal mucosa.
This type of chronic inflammation is marked by the formation of a granuloma, which is a cluster of macrophages surrounded by a ring of other cells, mainly lymphocytes. Macrophages can activate to form epithelioid cells (cells that resemble epithelial cells). Some of these cells may merge to create larger cells known as giant cells, which can include:
- Langerhans giant cells. These have nuclei located at the edge in a horse-shoe pattern and are found in tuberculosis.
- Foreign body giant cells. Here, the nuclei are arranged randomly and are found in granulomas resulting from foreign objects like sutures and talc.
- Touton giant cells. These appear in conditions like xanthomas and are formed from merging epithelioid cells, characterised by a ring of nuclei surrounded by foamy cytoplasm.
- Physiological giant cells. Seen in osteoclasts, syncytiotrophoblasts, and megakaryocytes.
Common conditions leading to granuloma formation include:
- Tuberculosis
- Sarcoidosis (non-caseating granuloma)
- Brucellosis
- Cat scratch disease (stellate-shaped or round granuloma)
- Syphilis (gummas)
- Lymphogranuloma inguinale
- Leprosy
- Inflammatory bowel disease (IBD)
The process of granuloma formation is explained further in the chapter on Immunity.
Understanding Wound Healing
- Wound healing is the process through which damaged tissue is either regenerated by similar cells or replaced with connective tissue.
- Regeneration involves the growth of new cells and tissues that completely restore the lost or damaged tissue.
- Repair is a combination of regeneration and scar formation, where collagen is deposited. While it can restore some original structures, it may disrupt the overall tissue architecture.
Healing by Primary Intention
Healing by primary intention, also known as first intention or primary union, refers to the process of healing a clean, uninfected wound. This type of healing involves several stages and changes over time.
Features of Wound Healing by Primary Intention
- Day 0: When the wound has just formed, a blood clot is present in the incision.
- Day 1 (within 24 hours): Neutrophils (a type of white blood cell) infiltrate the wound site, and the blood clot is still present.
- Day 2 (24 to 48 hours): Neutrophils continue to be present along with the blood clot, and a continuous thin epithelial layer begins to form over the wound.
- Day 3:
- Neutrophils are replaced by macrophages (another type of white blood cell).
- Granulation tissue, which is a sign of healing, starts to appear.
- Type III collagen deposition begins, but it does not yet bridge the incision.
- Day 5:
- Abundant granulation tissue is present.
- Collagen fibrils begin to bridge the incision.
- Neovascularization (the formation of new blood vessels) peaks.
- The epithelial layer reaches full thickness and undergoes surface keratinization.
- End of 2nd week: Collagen accumulates, and fibroblast proliferation occurs.
- 1 month:
- Collagen type III is replaced by collagen type I, which has greater tensile strength. This replacement is facilitated by the collagenase enzyme.
- Vitamin C plays a crucial role in this process by aiding the hydroxylation of lysine and proline residues, which stabilizes the collagen molecules.
Healing by Secondary Intention
- Healing by secondary intention involves a stronger inflammatory response, abundant granulation tissue, and the formation of a large scar.
- Over time, the size of the scar reduces due to a process called scar contraction.
Myofibroblasts are specialized fibroblasts with contractile properties due to smooth muscle microfilaments like alpha actin and vimentin in their cytoplasm. They can originate from fibroblasts, fibrocytes in the bone marrow, or epithelial cells.
- Wound strength is initially about 10% after one week, rapidly increasing to 70% by three months, with ongoing improvement over time.
- Type I collagen is predominant in adult skin, while early granulation tissue contains mainly type III collagen and some type I collagen.
- The balance of synthesis and breakdown of the extracellular matrix (ECM) is crucial for remodelling connective tissue in chronic inflammation and wound repair.
- Zinc-dependent matrix metalloproteinases (MMPs) facilitate collagen breakdown, essential for tissue remodelling, angiogenesis, and cancer spread. Zinc deficiency can impair wound healing.
- MMPs are produced by various cells, including fibroblasts, macrophages, and neutrophils.
- Tissue inhibitors of metalloproteinases (TIMPs) quickly inhibit activated collagenases to prevent uncontrolled protease activity, necessary for effective wound healing.
- Wound healing complications can stem from:
- Delayed healing due to foreign bodies, inadequate blood flow, diabetes, poor nutrition, hormonal factors (like glucocorticoids), infections, or scurvy.
- Deficient scar formation leading to issues like wound dehiscence and ulceration.
- Wound dehiscence, or rupture, is most common after abdominal surgery due to increased pressure.
- Granulation tissue comprises fibroblasts, small blood vessels, and chronic inflammatory cells like macrophages and lymphocytes.
- Neovascularization is prominent around day five.
- Granulation tissue is a crucial aspect of fibrogenic repair.
- The primary cell responsible for scar contraction is the myofibroblast.
- Zinc acts as a cofactor for certain enzymes, including matrix metalloproteinases.
- Infections are the leading cause of impaired wound healing.
- In human embryos, stem cells emerge in the yolk sac around the third week of development.
- In adults, most stem cells are found in the bone marrow, with some circulating in peripheral blood.
- Stem cells are utilized in bone marrow transplantation to treat various types of leukaemia and lymphoma.
- Excessive repair component formation can result from increased granulation tissue or collagen, leading to conditions such as keloids, hypertrophic scars, and 'proud flesh'.
- The sternum is the most common site for keloid formation.
- Intralesional steroids like triamcinolone are commonly used to manage keloids.
- Incisional scars or traumatic injuries may cause overgrowth of fibroblasts and connective tissue, known as desmoids or aggressive fibromatoses, which often recur after removal.
- Contractures are more likely to develop on the palms, soles, and front of the chest, particularly following severe burns.
Understanding Stem Cells
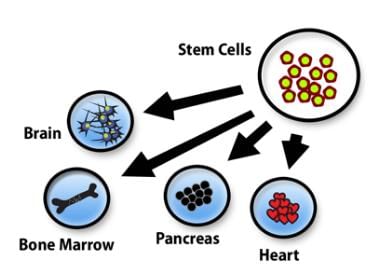
Definition: Stem cells are defined as cells capable of producing identical daughter cells (self-renewal) and developing into different specialized cell types (potency).
Self-renewal
Self-renewal can occur in two ways:
- Asymmetric cell division: This process generates one daughter cell that is identical to the parent cell and another that is different, becoming a progenitor or specialized cell. This method does not increase the total number of stem cells.
- Symmetric cell division: This type of division is necessary for growing stem cells in a laboratory setting. It involves the division of stem cells into two identical daughter cells, contributing to the increase in stem cell population.
It's important to note that self-renewal alone does not define stem cells. Established cell lines, such as HeLa cells, can also grow through symmetric division.
Potency
Potency refers to a cell's ability to differentiate into specific cell types, which can be classified as:
- Totipotent cells: These cells have the potential to develop into an entire organism. The zygote is the only cell with totipotent ability.
- Pluripotent cells: These cells, such as embryonic stem cells, can differentiate into all body tissues.
- Multipotent cells: These cells can give rise to multiple cell types but not all types found in the body. For example, hematopoietic stem cells can produce various blood cell types.
- Oligopotent cells: These cells can form more than one type of cell but fewer than multipotent cells.
- Unipotent cells:These cells can produce only a single type of specialized cell. Terminally differentiated cells, like fibroblasts, can grow and divide but lack the ability to differentiate into other cell types, so they are not classified as unipotent. Adult stem cells typically have a more limited differentiation potential, usually restricted to specific tissues.
Location of Stem Cells
Stem cells are located in specific areas known as niches within the body. Some examples of stem cell niches include:
- Oval cells: These stem cells are found in the canals of Herring in the liver. They have the potential to differentiate into hepatocytes (liver cells) and biliary cells.
- Satellite cells: Located in the basal lamina of myotubules in skeletal muscle, satellite cells play a crucial role in muscle regeneration. After injury, they can differentiate into myocytes (muscle cells) to repair damaged tissue.
- Limbus cells: These stem cells are situated in the canals of Schlemm in the eye. They are responsible for generating new corneal cells, contributing to the maintenance and repair of the cornea.
- Ito cells: Also known as hepatic stellate cells, Ito cells are found in the subendothelial space of Disse in the liver. They store vitamin A and play a role in liver regeneration and fibrosis.
- Paneth cells: Located at the base of intestinal crypts in the small intestine, Paneth cells are involved in the defense against microorganisms. They secrete antimicrobial substances and help maintain gut health.
- Stem cells are also present in other locations, such as the base of the crypts in the colon, where they contribute to the regeneration of intestinal lining cells, and the dentate gyrus of the hippocampus in the brain, where they are involved in neurogenesis (the formation of new neurons).
Other Significant Concepts in Stem Cell Biology
- Developmental Progression: Development starts with totipotent fertilised eggs, which are capable of forming any cell type. These cells then give rise to pluripotent epiblast cells, which can develop into many different cell types but not all. This process continues to multipotent cells, which are limited to a specific range of cell types, and finally to terminally differentiated cells, which have a specific function and cannot change into other cell types.
- Nuclear Reprogramming: This involves reverting terminally differentiated cells back to a totipotent or pluripotent state. This is done through a process called nuclear transplantation or cloning, where the nucleus of a differentiated cell is placed into an enucleated oocyte (an egg cell from which the nucleus has been removed).
- Stem Cell Plasticity and Trans-Differentiation: Traditionally, it was thought that once cells differentiate, they cannot change into other types. However, research has shown that tissue stem cells, which are multipotent and committed to a specific lineage, can actually differentiate into other cell types. For example, under certain conditions, hematopoietic stem cells (which usually form blood cells) might transform into neurons or germ cells. This ability to change is known as trans-differentiation.
|
53 docs|7 tests
|
FAQs on Inflammation Chapter Notes - Pathology - NEET PG
| 1. What is the difference between Myeloperoxidase deficiency and Chronic Granulomatous Disease (CGD)? |  |
| 2. What are the enzymes involved in the respiratory burst? |  |
| 3. What are the important actions of Nitric Oxide (NO) in the body? |  |
| 4. What are the functions of Bradykinin in the inflammatory response? |  |
| 5. How do Myeloperoxidase deficiency and CGD affect the immune system? |  |















