States of Matter Chapter Notes | Science Class 5 ICSE PDF Download
| Table of contents |

|
| Introduction |

|
| Solid |

|
| Liquid |

|
| Gas |

|
| Solubility of Substances in Water |

|
| Change of State |

|
| Separation of Liquids from Solids |

|
| Air |

|
| Points To Remember |

|
| Glossary |

|
Introduction
Everything around us that has weight and takes up space is called matter. This chapter teaches us about the different forms of matter, which are solids, liquids, and gases. We will learn how tiny particles called molecules and even smaller units called atoms make up matter. The chapter explains the properties of solids, liquids, and gases, how substances can dissolve in water, and how matter can change its form. It also covers how to separate substances and the role of air and ventilation in our lives.
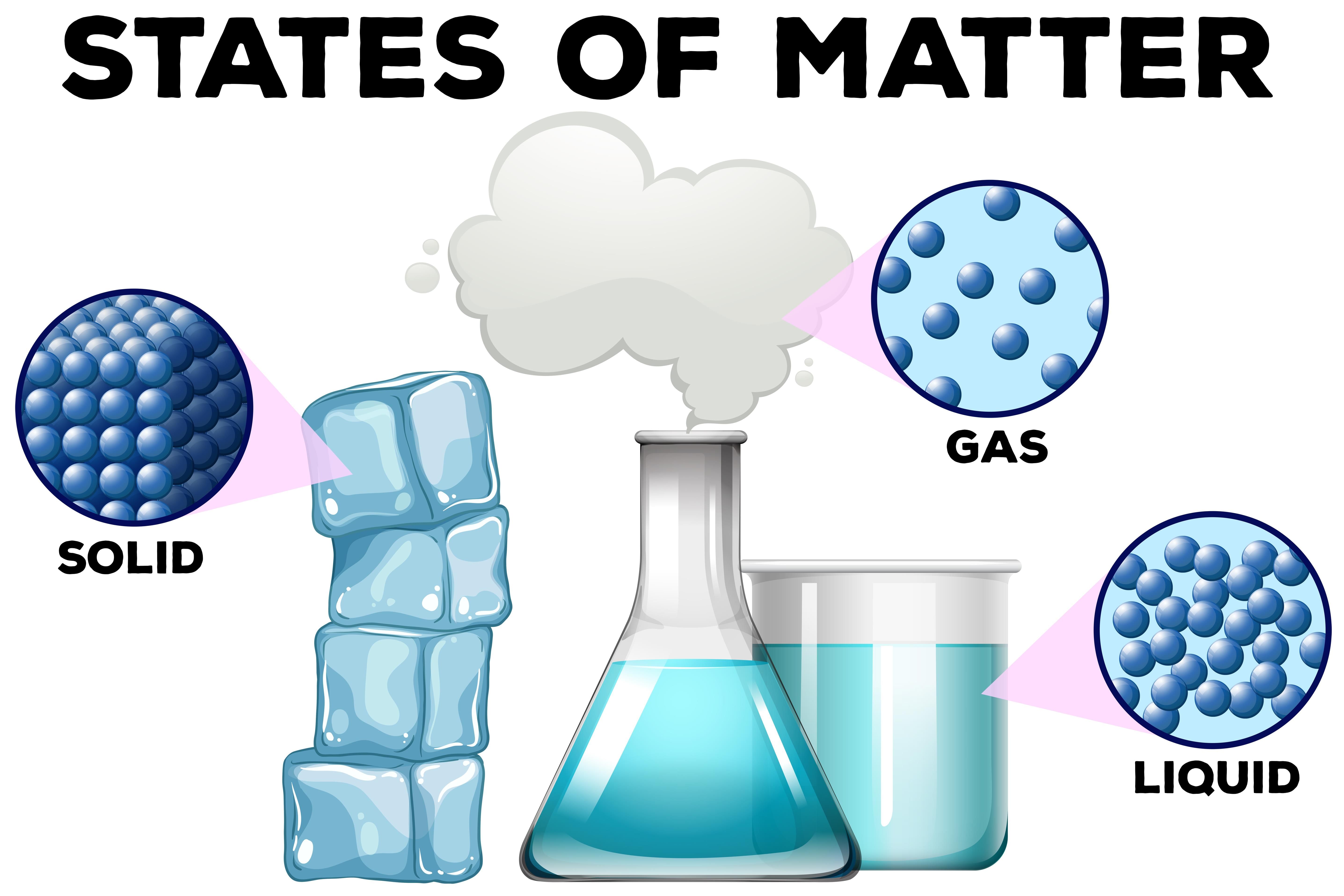
Solid
- Solids have a fixed shape and size because their molecules are packed very close together.
- The molecules in a solid have a strong force of attraction between them.
- Properties:
- Solids have a fixed shape that does not change easily.
- Solids have a fixed volume, which means they have a definite size.
- Solids take up a specific space and do not change their position easily.
- Solids cannot flow like liquids or gases.
- The shape of some solids can be changed by applying force, like bending or breaking.
- Molecules in some solids, like sugar crystals and diamonds, are arranged in a definite pattern.
- When solids with a definite pattern are broken, each piece still keeps the same pattern.
- Molecules in some solids, like rubber, glass, and plastic, are not arranged in a definite pattern.
Liquid
- Liquids have molecules that are not packed tightly, so they can move a little.
- The force of attraction between molecules in liquids is weaker than in solids.
- Properties:
- Liquids do not have a fixed shape; they take the shape of the container they are in.
- Liquids have a fixed volume, which means their amount can be measured.
- Liquids take up a specific space in a container.
- Liquids can flow easily from a higher level to a lower level.
- Examples of liquids are water, petrol, soft drinks, and milk.
Gas
- Molecules in gases are very loosely packed compared to solids and liquids.
- The force of attraction between molecules in gases is very weak, so they move freely in all directions.
- Properties:
- Gases do not have a fixed shape; we cannot see their shape.
- Gases do not have a fixed volume; they spread out to fill any space.
- Gases have very little weight compared to solids and liquids.
- Gases can move freely in any direction and take up all the space available to them.
- Air is made up of gases like nitrogen, oxygen, and carbon dioxide.
- Oxygen and carbon dioxide are important gases for life on Earth.
- We can show that gas takes up space by doing an activity with a glass, bucket, and water.
Solubility of Substances in Water
- Solubility is the ability of a substance to dissolve in another substance to form a mixture.
- The substance that dissolves is called the solute.
- The substance in which the solute dissolves is called the solvent.
- The mixture formed after dissolving is called a solution.
- Water is a very good solvent because many substances dissolve in it, so it is called the universal solvent.

Solubility of Solids in Water
- Some solids dissolve in water, like salt and sugar, which are common examples.
- Some solids do not dissolve in water, like sand and chalk powder; they settle at the bottom.
Solubility of Liquids in Water
- Many liquids dissolve easily in water, which can be seen by doing a simple activity.
- Take a mug of water and add a few drops of ink; the ink dissolves quickly, and the water becomes colored.
- Fruit juices and milk also dissolve in water and mix well.
- Some liquids do not dissolve in water, like petrol, kerosene oil, and butter; they are immiscible in water.
- Liquids that dissolve completely in water are called miscible.
- Liquids that do not dissolve in water are called immiscible.
Solubility of Gases in Water
- Gases can also dissolve in water, and this can be seen in carbonated drinks like soda.
- In carbonated drinks, carbon dioxide gas is dissolved in water under pressure.
- When you open a soda bottle, the pressure decreases, and the gas comes out as bubbles.
- Other gases like oxygen and nitrogen also dissolve in water, which helps aquatic animals breathe.
Did You Know?
- Carbonated drinks are aerated drinks like colas and drinking soda.
- These drinks have carbon dioxide gas dissolved in them under pressure.
Change of State
- Matter can change its form from one state to another, like solid to liquid or liquid to gas.
- The change of state happens because the arrangement of molecules in the matter changes.
- Changes of state can be of two types: physical change and chemical change.
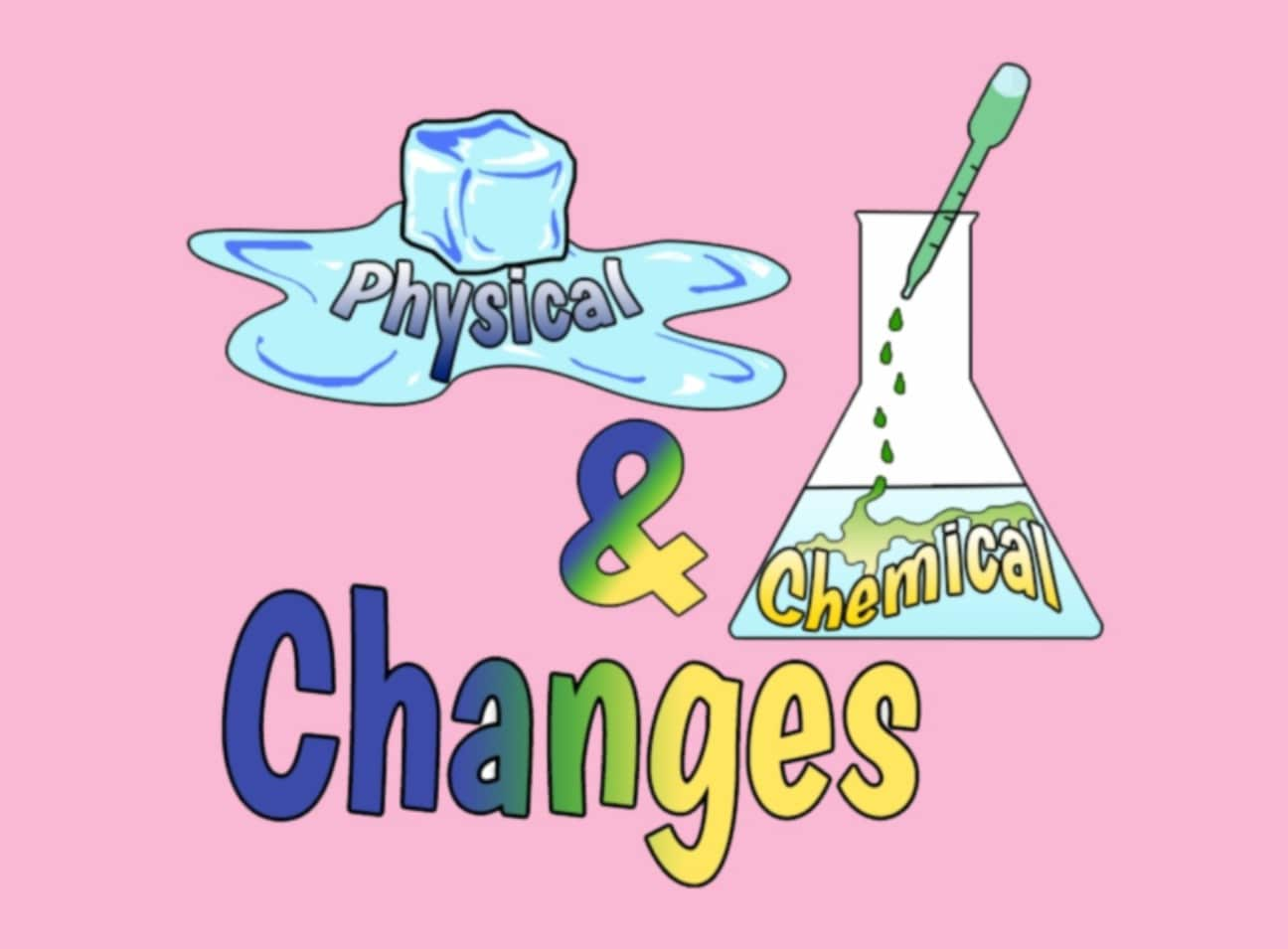
Physical Change
- A physical change is when no new substance is formed, and the change can usually be reversed.
- For example, when water cools, it becomes ice (solid), which is a physical change.
- When ice is heated, it melts back into water (liquid), showing the change can be reversed.
- Another example is when water is heated, it becomes steam (gas), and when steam cools, it turns back into water.
Chemical Change
- A chemical change is when one or more new substances are formed, and the change cannot be reversed.
- For example, burning wood forms ash, which is a new substance, and we cannot get the wood back.
- Other examples of chemical changes are rusting of iron and digestion of food in our body.
Separation of Liquids from Solids
- We can separate solids that dissolve in liquids and solids that do not dissolve using different methods.
- Solids like salt and sugar dissolve in water, while solids like mud and sand do not dissolve.
- Some solids float on water if they are lighter, while others settle at the bottom if they are heavier.
- We use different techniques to separate these solids from liquids.
Separating Soluble Solids
Soluble solids are solids that dissolve in water, like salt and sugar. Two simple methods to separate soluble solids from water are evaporation and distillation.
- In evaporation, heat a solution of salt and water in a vessel until the water turns into vapor, leaving the salt behind.
- Distillation is another method that uses a special setup called a distillation unit.
- The distillation unit has a round-bottomed flask, a water condenser, a receiving flask, and a burner.
- In distillation, heat the solution in the round-bottomed flask until it boils.
- The boiling water turns into steam, which goes through the condenser and cools down to become liquid water again.
- The cooled water collects in the receiving flask, while the soluble solid stays in the round-bottomed flask.
Separating Insoluble Solids
- Insoluble solids are solids that do not dissolve in water, like sand and mud.
- Insoluble solids can be separated by methods like sedimentation, decantation, and filtration.
- Sedimentation and decantation are used for solids heavier than water.
- In sedimentation, let the heavy particles settle at the bottom of the container.
- In decantation, pour the clear water from the top into another container, leaving the settled solids behind.
- Filtration is a better method for separating very small insoluble solids.
- In filtration, pour the mixture through a filter paper in a funnel.
- The filter paper acts like a sieve, allowing water to pass through while keeping the small insoluble solids behind.
Air
- Air is a mixture of gases that surrounds us.
- The two main gases in air are nitrogen and oxygen.
- Nitrogen is the largest part of air, and oxygen is the second largest part.
- Air also has small amounts of other gases like carbon dioxide and water vapor.
- Other things in air include smoke, dust, and germs.
- Warm air is lighter than cold air, so warm air rises up, and cold air takes its place.
- The continuous movement of air from hot to cold regions is called air currents.
- In coastal areas, air currents cause sea and land breezes.

Sea and Land Breeze
- Land heats up and cools down faster than the sea because of the sun's heat.
- During the day, the land gets hotter than the sea, so the hot air above the land rises.
- The cooler air from the sea blows in to take the place of the hot air; this is called a sea breeze.
- At night, the land cools faster than the sea, so the air above the sea is warmer.
- The cooler air from the land blows towards the sea to replace the warm air; this is called a land breeze.
Monsoon Breeze
- During summer, both the land and the sea heat up due to the sun's rays.
- The land heats up more quickly, so the air above the land gets warmer than the air above the sea.
- The sea also warms up but stays cooler than the land.
- The air above the sea holds moisture, which is called the moist monsoon breeze.
- The warm air above the land rises, and the moist air from the sea rushes in to take its place.
- As the moist air cools over the land, the moisture in it turns into rain.
Role of Ventilators
- Ventilation is the process of replacing foul air inside a room with fresh air from outside.
- Ventilators and exhaust fans are used in homes to help with ventilation.
- Ventilation removes unwanted smells, like those from cooking or cleaning, from the house.
- The air we breathe out is warm and has carbon dioxide, which is impure air.
- Warm air is lighter, so it rises up, and fresh air enters the room through doors and windows.
- As the air pressure inside the room increases, the warm air goes out through the ventilators.
- Ventilation replaces the impure air with fresh air that is rich in oxygen, which we need to breathe.
- Ventilation also removes excess moisture from the air, keeping walls and furniture dry.
- Too much moisture in the air can cause mould or fungi to grow, which is bad for health.
- Mould can cause infections and damage things in the house, so we need to keep our homes well-ventilated.
- Ventilators are usually small windows without doors, placed at the top of walls in rooms.
- Sometimes exhaust fans are installed in these small windows to push out foul air.
- The foul air goes out, and fresh air comes in through open doors and windows for proper ventilation.
Points To Remember
- Matter is divided into solids, liquids, and gases based on how their molecules are arranged.
- Solids have a fixed shape, fixed volume, and cannot flow.
- Liquids do not have a fixed shape but have a fixed volume, and they can flow.
- Gases do not have a fixed shape or fixed volume; they move freely and spread out in any direction.
- Water is known as the universal solvent because many substances dissolve in it easily.
- Two simple ways to separate soluble solids from water are evaporation and distillation.
- Insoluble solids can be separated from water by methods like sedimentation, decantation, and filtration.
- Warm air is lighter than cold air, so warm air rises, and cold air takes its place.
- In coastal areas, air currents are responsible for causing sea and land breezes.
- Ventilation is the process of replacing foul air with fresh air inside a room.
Glossary
- Matter: Anything that has mass and takes up space.
- Solubility: The ability of a substance to dissolve in another substance to form a mixture.
- Solute: The substance that dissolves in a solvent to form a solution.
- Solvent: The substance in which the solute dissolves to form a solution.
- Physical Change: A change in matter where no new substance is formed, and the change can be reversed.
- Chemical Change: A change in matter where one or more new substances are formed, and the change cannot be reversed.
- Sedimentation: The settling down of heavy particles at the bottom of a container.
- Decantation: Pouring the clear water at the top of a jar into another jar after heavy particles settle down.
- Ventilation: The continuous replacement of foul air with fresh air inside a room.
|
60 docs|11 tests
|
FAQs on States of Matter Chapter Notes - Science Class 5 ICSE
| 1. What are the different states of matter? |  |
| 2. How does temperature affect the state of matter? |  |
| 3. What is solubility, and how does it relate to substances in water? |  |
| 4. What are some methods to separate liquids from solids? |  |
| 5. What role does air play in our daily lives? |  |




















