Matter Chapter Notes | Chemistry for Grade 11 PDF Download
Introduction
Hello, friends! Today, we are going to learn about something called "matter." Matter is everything around us that takes up space and has weight. It can be the toys you play with, the water you drink, or even the air you breathe! Matter is made of tiny, tiny pieces called atoms, and these atoms join together to make bigger pieces called molecules. Matter can look different and act different depending on how these tiny pieces move and stick together. We will learn that matter comes in three types: solids, liquids, and gases. We will also find out how matter changes from one type to another and why it stays the same in some ways even when it changes. Let’s dive into this fun chapter and explore the world of matter!
Matter
- Matter is anything that has weight and takes up space.
- Matter is made of tiny pieces called atoms.
- Atoms cannot live alone, so they join together to make bigger pieces called molecules.
- We already know that matter comes in three types:
- Solids: They have a fixed shape and size. They don’t change their shape when you move them.
- Liquids: They have a fixed size but not a fixed shape. They take the shape of the container you put them in.
- Gases: They don’t have a fixed shape or size. They spread out to fill the container they are in.
- Matter can be a solid, liquid, or gas depending on how hot or cold it is and how much it is squeezed.
Kinetic Molecular Theory of Matter
- The kinetic molecular theory tells us why matter is a solid, liquid, or gas and how it changes from one type to another.
- It says matter is made of tiny pieces that are always moving and have energy.
- The space between the tiny pieces and how strongly they stick together decides what type of matter it is.
Postulates of Kinetic Molecular Theory of Matter
- Matter is made of lots of tiny pieces that can be atoms or molecules.
- These tiny pieces are always moving, so they have energy called kinetic energy.
- The tiny pieces bump into each other and other things, and when they bump, they share energy, but they don’t lose any energy.
- Matter can change from one type to another if the energy of the tiny pieces changes.
- There are empty spaces between the tiny pieces called intermolecular spaces.
- The tiny pieces stick to each other because of a force called the intermolecular force of attraction.
- The space between tiny pieces gets smaller if there is more attraction between them.
- The stickiness between tiny pieces depends on what they are made of.
- Tiny pieces of the same kind stick together strongly with a force called cohesion.
- Tiny pieces of different kinds stick together with a force called adhesion.
States of Matter Based on Kinetic Molecular Theory
The type of matter depends on how much the tiny pieces stick together and how much space is between them. The kinetic molecular theory explains how matter acts in solids, liquids, and gases.
Solids
- In solids, the tiny pieces are very close to each other.
- The stickiness between the tiny pieces is very strong in solids.
- The tiny pieces are packed in a neat pattern and don’t move much, they just shake a little.
- Because the stickiness is strong, the tiny pieces don’t have enough energy to move away.
- So, the tiny pieces in solids only shake in their place and don’t move around.
Liquids
- In liquids, the tiny pieces are not as close as in solids but closer than in gases.
- The space between the tiny pieces in liquids is more than in solids but less than in gases.
- The stickiness between the tiny pieces in liquids is weaker than in solids but stronger than in gases.
- The tiny pieces in liquids can move around a little because they are not stuck as tightly.
- The energy of the tiny pieces is not strong enough to break the stickiness completely.
- But if you add a little energy, the tiny pieces can move away from each other.
- That’s why liquids can turn into gases when they get hot.
Gases
- In gases, the tiny pieces are very far apart compared to solids and liquids.
- The space between the tiny pieces in gases is the biggest.
- The stickiness between the tiny pieces in gases is very weak, almost nothing.
- The tiny pieces in gases move around a lot because they have a lot of energy.
- The energy of the tiny pieces is strong enough to break the stickiness, so they move freely.
States of Matter
Changing matter from one type to another depends on the space between tiny pieces and how much they stick together. It also depends on how much the tiny pieces bump into each other.
Let’s learn about solids, liquids, and gases in detail:
Solids
- In solids, the tiny pieces are very close and tightly packed.
- Solids have a fixed shape and size.
- The size of a solid depends on how big the tiny pieces are.
- The stickiness between tiny pieces in solids is very strong, so they don’t move much.
- Because of this, solids don’t have enough energy to move away from each other.
- The energy of the tiny pieces in solids depends on how hot or cold the solid is.
- The space between the tiny pieces in solids is very small, almost nothing.
Liquids
- In liquids, the tiny pieces can move around a little inside the liquid.
- Liquids are made of tiny pieces that are loosely packed and can move randomly.
- Liquids can keep their size but not their shape.
- The size of a liquid depends on how big the tiny pieces are.
- When the tiny pieces move, it changes how the liquid acts.
- Liquids have a fixed size and take the shape of the container they are in.
- The stickiness between tiny pieces in liquids is weaker than in solids but stronger than in gases.
- The energy of the tiny pieces in liquids depends on how hot or cold the liquid is.
- The space between tiny pieces in liquids is more than in solids but less than in gases.
- The tiny pieces in liquids are not as tightly packed as in solids.
Gases
- In gases, the tiny pieces are always moving and bumping into each other.
- Gases are made of tiny pieces that have a little stickiness but a lot of energy.
- The energy in gases is much more than in solids or liquids.
- The stickiness between tiny pieces in gases is very weak, so they spread out easily.
- Gases are made of lots of tiny pieces moving in all directions.
- The space between tiny pieces in gases is much bigger than in solids and liquids.
- Since the tiny pieces are small, the space they take up is also small compared to the whole space.
- The tiny pieces in gases don’t stick to each other much, so they move far apart.
- The energy of the tiny pieces in gases depends on how hot or cold the gas is.
- The space between tiny pieces in gases is the biggest.
Change of State of Matter
- Matter can change from one type to another, like from solid to liquid, and this is called a change of state.
- Changing the state depends on how much the tiny pieces move and how much they stick together.
- When a solid gets hot, the tiny pieces get more energy and start moving more.
- The tiny pieces in a solid try to break the stickiness between them when they get more energy.
- So, they shake faster and the space between them gets bigger.
- As the space grows and the stickiness gets weaker, the solid turns into a liquid.
- When the liquid gets hotter, the tiny pieces get even more energy.
- The tiny pieces in the liquid move faster, and the space between them grows more.
- The stickiness between the tiny pieces gets very weak, and the liquid turns into a gas.
- When a gas cools down, the tiny pieces lose energy and slow down.
- The stickiness between the tiny pieces gets stronger, and they come closer together.
- The space between the tiny pieces gets smaller, and the gas turns into a liquid.
- If the liquid cools more, the tiny pieces slow down even more, and the stickiness gets stronger.
- The space between the tiny pieces gets very small, and the liquid turns into a solid.
Terms Related to Change of State of Matter
- Boiling: When a liquid turns into a gas at a certain temperature, it’s called boiling.
- Freezing: When a liquid turns into a solid by cooling, it’s called freezing.
- Melting: When a solid turns into a liquid at a certain temperature, it’s called melting.
- Condensation: When a gas turns into a liquid, it’s called condensation.
- Sublimation: When a solid turns straight into a gas without becoming a liquid, it’s called sublimation.
- Deposition: When a gas turns straight into a solid without becoming a liquid, it’s called deposition.
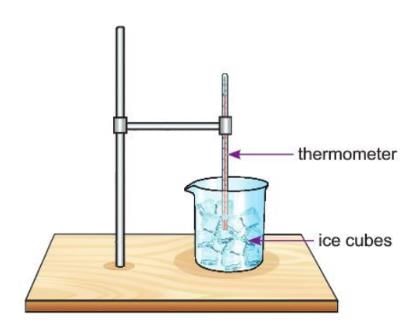
Activity 1.1
Aim: To observe change when ice melts
Materials required: A glass beaker, some ice cubes, thermometer.
Procedure:
- Take some ice cubes and put them in a glass beaker.
- Use a thermometer to check the temperature of the ice every minute.
- Draw a graph of the temperature and time on graph paper.
- Write down what you see in a table.
Observation: As the temperature goes up, the ice starts to melt and turns into liquid water.
Law of Conservation of Mass
- The law of conservation of mass says that matter cannot be made or destroyed. The amount of matter stays the same even when it changes from one type to another.
- The size, energy, or shape of matter can change, but the total amount of matter stays the same. This happens because the tiny pieces in matter are not created or destroyed, they just change how they are arranged.
Let's understand the law of conservation of mass with the help of some examples:
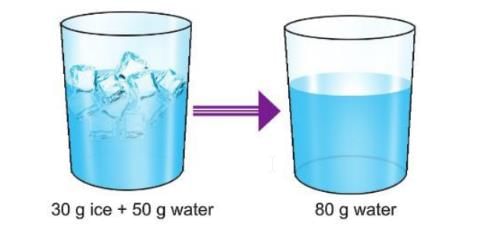
- Example 1: When you mix 30 g of ice with 50 g of water, the total is 30 g + 50 g = 80 g. After the ice melts, you get 80 g of water, so the amount stays the same.
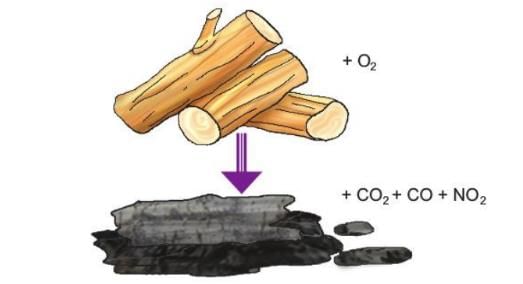
- Example 2: When wood burns in air, it turns into ash, but the ash weighs less than the wood. But if you add the weight of the wood and the air it used, it equals the weight of the ash and the gases made, like CO₂, NO₂, and water vapor. So, the total amount before burning equals the total amount after burning.
- Example 3: When you burn magnesium ribbon, it turns into magnesium oxide, which is a white solid. The magnesium (2Mg) and oxygen (O₂) together weigh the same as the magnesium oxide (2MgO) made.
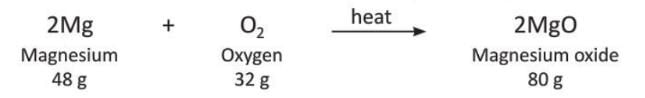
In all these examples, the total amount of matter before and after a change is the same. During a change, the tiny pieces break apart and join in new ways, but the total amount doesn’t change.
Did You Know?
Antoine Lavoisier, in the late 18th century, discovered the Law of Conservation of Mass. He called this law a big rule in science.
Activity 1.2
Aim: To show that the total mass remains same during a physical changeMaterials required: An ice cube, sealable plastic bag, measuring scale.
Procedure:
- Take a sealable plastic bag and put an ice cube in it.
- Measure the weight of the ice cube and the plastic bag together.
- Wait until the ice cube melts into water.
- Measure the weight of the plastic bag with the water in it again.
- Compare the weight of the plastic bag with the ice cube and after the ice cube melts.
- Write down what you see.
Observation: The ice cube changes from solid to liquid, but the weight stays the same.
Conclusion: This shows that when matter changes from one type to another, the total amount doesn’t change.
Activity 1.3
Aim: To show that the total mass remains same during a chemical changeMaterials required: 20 ml vinegar, 2 cups, a sealable plastic bag.
Procedure:
- Take 20 ml of vinegar in a cup and measure the weight of the cup with the vinegar.
- Take 10 g of baking soda in another cup and put it in a sealable plastic bag.
- Measure the weight of the plastic bag with the baking soda in it.
- Put the cup with vinegar inside the same plastic bag as the baking soda cup.
- Be careful, open the seal of the bag, and pour the vinegar into the cup with baking soda.
- Now measure the weight of the plastic bag with everything in it.
- Write down what you see and compare the weight before and after mixing.
Observation: The vinegar and baking soda mix and make a new thing, but the weight stays the same.
Conclusion: This shows that even when matter changes in a chemical way, the total amount stays the same. Total weight of the starting things = Total weight of the new things made.
Activity 1.4
Aim: To show that the total mass of reactants is equal to the total mass of products in a chemical reaction between barium chloride and sodium sulphate
Materials required: A conical flask, a test tube, cork, barium chloride, sodium sulphate, measuring scale.
Procedure:
- Take a conical flask and make a mix of barium chloride and water in it.
- Take a test tube and make a mix of sodium sulphate in it.
- Put the test tube with the sodium sulphate mix into the conical flask with the barium chloride mix carefully.
- Close the conical flask with a cork tightly.
- Measure the weight of the flask and everything in it using a measuring balance.
- Now lift the flask, turn it upside down carefully, and mix the two things together.
- The sodium sulphate mix pours out of the test tube and mixes with the barium chloride mix in the flask.
- The mix makes barium sulphate and sodium chloride.
- The things that mix are: Barium chloride + Sodium sulphate.
- The new things made are: Barium sulphate + Sodium chloride.
- Measure the weight of the flask and everything in it again after mixing.
Observation: The weight of the flask before mixing is the same as the weight after mixing.
Conclusion: This shows that the total weight of the starting things is the same as the total weight of the new things made in a chemical change.
Note: Barium chloride is a dangerous chemical, and touching it should be avoided.
Points To Remember
- Matter is anything that has weight and takes up space.
- Solids, liquids, and gases are the three types of matter.
- Liquids have a fixed size, and they take the shape of the container they are poured into.
- Gases don’t have a fixed shape or size; they spread out to fill the container they are in.
- The kinetic molecular theory says matter is made of tiny pieces that are always moving and have energy.
- There is a stickiness between tiny pieces, and there is space between them too.
- The type of matter depends on how much the tiny pieces stick together and the space between them.
- The kinetic molecular theory explains how matter acts in different types.
- The law of conservation of mass says that matter cannot be made or destroyed.
- The amount of matter and the space it takes up can change, but the total amount stays the same because tiny pieces are not created or destroyed.
- Matter can change its shape, energy, and other things during a change, but the total amount of matter stays the same.
- We can say that the total amount of starting things equals the total amount of new things in a chemical change.
Glossary
- Matter: Anything that has weight and takes up space.
- Kinetic molecular theory of matter: A big idea that says matter is made of tiny pieces that move and have energy.
- Intermolecular force of attraction: The stickiness between tiny pieces of matter.
- Intermolecular space: The empty space between tiny pieces of matter.
|
68 docs|10 tests
|
FAQs on Matter Chapter Notes - Chemistry for Grade 11
| 1. What is the Kinetic Molecular Theory of Matter? |  |
| 2. How does the Kinetic Molecular Theory relate to the states of matter? |  |
| 3. What are the changes of state of matter? |  |
| 4. What is the Law of Conservation of Mass? |  |
| 5. How can I summarize the properties of the three states of matter? |  |