Elements, Compound and Mixtures Chapter Notes | Chemistry Class 8 ICSE PDF Download
| Table of contents |

|
| Introduction |

|
| Elements, Compounds and Mixtures—A Quick Recall |

|
| Pure Substances |

|
| Mixtures |

|
| Separation of Mixtures |

|
| Points To Remember |

|
| Glossary |

|
Introduction
Hey kids! Today we are going to learn about something super fun in science—elements, compounds, and mixtures! Imagine you have a big box of toys. Some toys are made of just one kind of piece, like a single Lego block—that’s like an element. Some toys are made by sticking two Lego blocks together in a special way—that’s like a compound. And sometimes, you mix lots of toys together, like blocks, dolls, and cars—that’s a mixture! In this chapter, we will find out what elements, compounds, and mixtures are, how they are different, and how we can separate them. Let’s dive into this exciting world of science!
Elements, Compounds and Mixtures—A Quick Recall
- Everything around us that has weight and takes up space is called matter.
- Matter can be divided into two big groups: pure substances and mixtures.
- Pure substances are things like elements and compounds.
- Mixtures are when two or more things are mixed together.
Pure Substances
A pure substance is something that has the same kind of pieces and the same special features all over. We can separate a pure substance into smaller pieces using easy methods like breaking or melting.
Characteristics of Pure Substances
- A pure substance is always made of the same kind of pieces.
- For example, a piece of carbon dioxide always has one carbon piece and two oxygen pieces—we can’t change this number.
- A pure substance has special melting and boiling points. It also has a special taste, color, or smell that doesn’t change.
Elements
- Elements are pure substances made of only one kind of atom—the tiniest piece of matter. We know about 118 elements in nature, and some are made by scientists too. Elements can be solid, liquid, or gas, and we divide them into metals, non-metals, metalloids, and noble gases based on their special features.
- Metals are shiny, can carry heat and electricity, can be shaped, and make a sound when hit. Examples are gold, silver, iron, and aluminum.
- Non-metals are not shiny, don’t carry heat or electricity well, and don’t make a sound. Examples are oxygen, chlorine, and nitrogen.
- Metalloids have some features of metals and some of non-metals. Examples are germanium, arsenic, silicon, boron, antimony, tellurium, and bismuth.
- Noble gases don’t like to mix with other elements because they are very lazy. Examples are helium, neon, argon, krypton, and radon.
Compounds
- A compound is a pure substance made when two or more elements join together in a fixed amount.
- We can break a compound into its elements using special science reactions.
- The features of a compound are very different from the elements it is made of.
- For example, water is a compound made of hydrogen and oxygen, but water’s features are not like hydrogen or oxygen.
Mixtures
A mixture is when two or more pure substances, like elements or compounds, are mixed together but keep their own special features. For example, air is a mixture of gases like nitrogen, oxygen, carbon dioxide, and water vapor.
Characteristics of Mixtures
- A mixture doesn’t have a fixed amount of its pieces—the amounts can change.
- We can separate a mixture into its pieces using easy methods because the pieces don’t stick together strongly.
- The pieces in a mixture keep their own special features.
- A mixture doesn’t have fixed melting or boiling points.
- No energy is used or given off when a mixture is made.
Classification of Mixtures
Mixtures can be homogeneous or heterogeneous:
- A homogeneous mixture is when all the pieces are mixed evenly, like salt in water, sugar in water, or an alloy like iron and carbon.
- A heterogeneous mixture is when the pieces are not mixed evenly, like oil in water or chalk in water—they can be seen separately. Heterogeneous mixtures can be suspensions or emulsions.
- A suspension is a mixture where one piece doesn’t fully mix and settles down, like chalk in water or dust in air.
- An emulsion is a mixture of two liquids that don’t mix, like oil and water—they form tiny drops in each other.
Formation of Mixtures
Mixtures can be in different forms depending on the state of their pieces.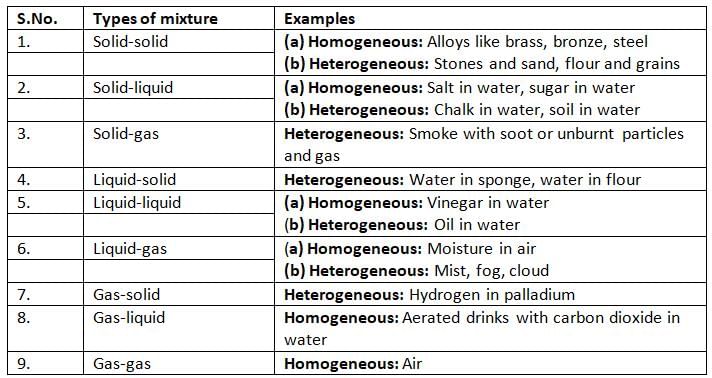
Separation of Mixtures
- Mixtures are made of two or more pure substances that can come in many forms.
- We can separate mixtures to get useful things, make pure substances, or remove bad stuff.
- We separate mixtures because:
- To get a useful thing from a mixture, like getting iron nails from sand.
- To get a pure substance and make it even purer, like cleaning salt from seawater.
- To remove harmful or unwanted things, like cleaning impurities from water.
Principle of Separation of Mixtures
Each piece in a mixture has its own special features, like weight, size, or chemical behavior. We use these differences to separate the pieces of a mixture:
Separating Solid-Solid Mixtures
Handpicking
- Handpicking is when we pick pieces of a mixture with our hands.
- It works when the pieces are different in size, shape, or color, and easy to see.
- For example, we can pick small stones from rice or pulses using handpicking.
Sieving
- Sieving is when we use a sieve to separate pieces of a mixture based on their size.
- A sieve has small holes, so smaller pieces fall through, and bigger pieces stay on top.
- For example, we can sieve sand to remove small stones at construction sites.
- Sieving is also used to remove impurities from flour.
Activity 3.1
Aim: To separate the components of a mixture by sieving.
Materials required: Some flour, some pulses, container, sieve.
Procedure:
- Take some flour and pulses and mix them.
- Take a container and place a sieve over it.
- Put the mixture of flour and pulses on the sieve.
- Shake the sieve over the container.
Observation: The flour passes through the holes in the sieve into the container, while the pulses stay on the sieve.
Conclusion: This shows that a mixture with pieces of different sizes can be separated by sieving.
Winnowing
- Winnowing is when we use wind or moving air to separate pieces of a mixture.
- It works because the pieces have different weights.
- The lighter pieces are blown away by the wind, and the heavier pieces fall down.
- For example, farmers use winnowing to separate grains from husk and chaff.
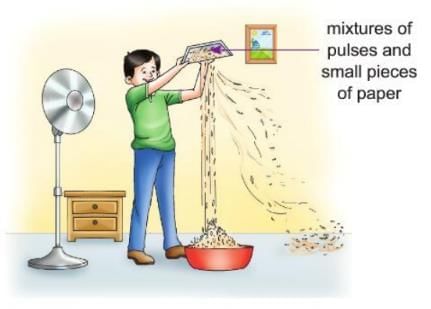
Activity 3.2
Aim: To separate the components of a mixture by winnowing.
Materials required: Some gram, small bits of paper, a tray, a tub, a pedestal fan.
Procedure:
- Take some gram and mix it with small bits of paper.
- Take a tray and put the mixture in it.
- Place a large tub on the floor.
- Place a pedestal fan near the tub and switch it on.
- Stand in front of the fan, as shown in the figure, and hold the tray high above the tub.
- Allow the mixture to fall slowly into the tub while the fan blows air.
Observation: The bits of paper are blown away, and the gram falls into the tub.
Conclusion: This shows that pieces of a mixture with different weights can be separated by winnowing.
Magnetic Separation
- Magnetic separation is when we use a magnet to separate pieces of a mixture.
- It works because some pieces, like iron, are attracted to the magnet, while others, like sand, are not.
- For example, we can separate iron filings from sand using a magnet.
Gravitation
- Gravitation is when we use the weight of pieces to separate a mixture.
- It works when one piece is lighter than water and the other is heavier.
- For example, we can separate sawdust and sand by putting them in water—sawdust floats, and sand settles down.
- We can then separate the sawdust from water by filtering.
Solvent Extraction
- Solvent extraction is when we use a liquid to separate pieces of a mixture based on how well they dissolve.
- For example, we can separate sodium chloride (salt) and calcium carbonate from a mixture using water.
- Salt dissolves in water, but calcium carbonate does not.
- We put the mixture in water, stir it, and filter it—salt goes with the water, and calcium carbonate stays behind.
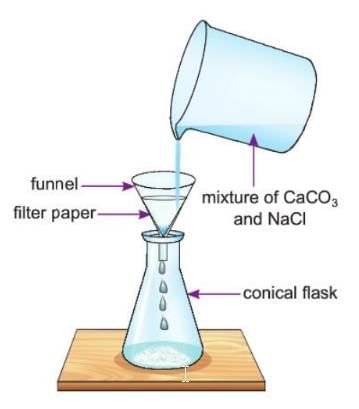
Activity 3.3
Aim: To separate the components of a mixture of calcium carbonate and sodium chloride.
Materials required: Mixture of calcium carbonate (CaCO₃) and sodium chloride (NaCl), beaker, filter paper, conical flask, funnel, water.
Procedure:
- Take the mixture of calcium carbonate (CaCO₃) and sodium chloride (NaCl) in a beaker.
- Pour some water into the beaker and stir it well until all the salt (NaCl) dissolves. Add more water if needed.
- Use a filter paper, make a cone, and place it in a funnel.
- Pour the contents of the beaker into the funnel.
- Separate the NaCl from water by heating the conical flask to dry it up.
Observation: Calcium carbonate (CaCO₃) stays on the filter paper, and some remains in the beaker. The flakes of sodium chloride (NaCl) are found in the conical flask after evaporation.
Conclusion: This shows that calcium carbonate and sodium chloride can be separated by filtration and evaporation because calcium carbonate doesn’t dissolve in water, but sodium chloride does.
Fractional Crystallisation
- Fractional crystallisation is when we separate pieces of a mixture by how well they dissolve in the same liquid.
- For example, we can separate common salt and potassium nitrate from a mixture.
- Both dissolve in water, but potassium nitrate dissolves more than salt.
- When the water cools, potassium nitrate turns into crystals first, leaving salt behind.
Sublimation
- Sublimation is when a solid turns into a gas without becoming a liquid first.
- We can use sublimation to separate a mixture if one piece can sublimate, like iodine or camphor.
- For example, to separate iodine and sand, we heat the mixture—iodine turns into a gas and leaves the sand behind.
- When the gas cools, it turns back into solid iodine.
Separating Solid-Liquid Mixtures
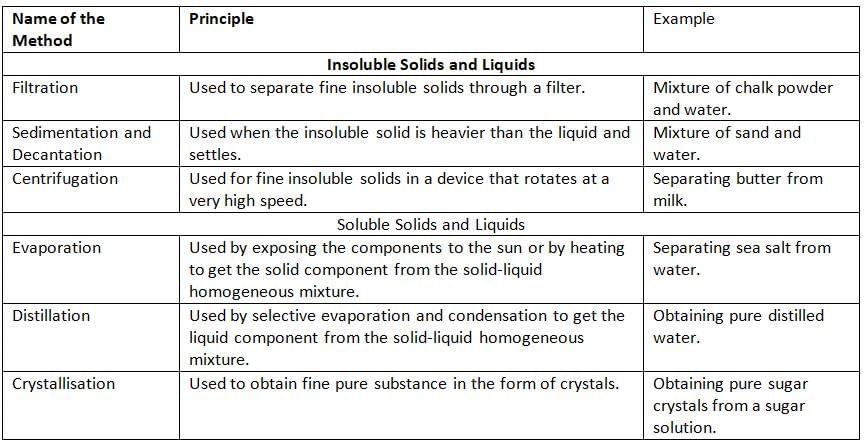
Sedimentation and Decantation
- Sedimentation is when we let a mixture of solid and liquid sit so the heavy solid settles down at the bottom.
- The solid that settles down is called sediments.
- The clear liquid on top of the sediments is called supernatant.
- Decantation is when we carefully pour the clear liquid (supernatant) into another container without disturbing the sediments.
- Example: If we mix sand and water, sand is heavy and settles at the bottom. We pour the water into another cup, leaving the sand behind.
Filtration
- Filtration is a way to separate a solid from a liquid by pouring the mixture through a filter.
- The filter can be filter paper, sand, or charcoal.
- The filter lets the liquid pass through but stops the solid.
- The solid left on the filter is called residue.
- The liquid that passes through the filter is called filtrate.
- Example: If we mix chalk powder and water, we can pour it through filter paper. The chalk stays on the paper, and water goes through.
- We can separate mixtures like tea and tea leaves, chalk and water, clay and water, or sawdust and water using filtration.
Evaporation
- Evaporation is when we heat a liquid so it turns into vapor (gas) and leaves the solid behind.
- We can separate a solid that is mixed in a liquid by heating the mixture.
- The liquid turns into vapor, and the solid stays behind.
- Example: If we mix salt and water, we can heat it. The water turns into vapor and goes away, leaving the salt behind.
Crystallisation
- Crystallisation is a way to get a pure solid from a liquid by making crystals.
- We heat a mixture of a solid and liquid, then let it cool slowly.
- When it cools, the solid forms shiny crystals with a perfect shape.
- Example: If we heat sugar and water, then cool it slowly, we get shiny sugar crystals.
Froth Flotation
- Froth flotation is a way to separate a special solid (called ore) from another solid (called gangue).
- We mix the solids with a liquid (like pine oil) and water, then blow air into the mixture.
- The air makes bubbles, and the ore sticks to the bubbles and floats up as froth.
- The gangue is heavier, so it settles down at the bottom.
- We can then dry the froth to get the ore.
- Example: We can separate ores of zinc, lead, or copper from gangue using froth flotation.
Distillation
- Distillation is a way to separate a solid from a liquid by heating and cooling.
- We heat the mixture in a special container called a distillation flask.
- The liquid turns into vapor, and the vapor goes through a condenser, which cools it back into a liquid.
- The solid stays behind in the distillation flask.
- The pure liquid is collected in a conical flask.
- Example: We can get pure distilled water from a mixture using distillation.
Centrifugation
- Centrifugation is a way to separate tiny solids from a liquid by spinning the mixture very fast.
- We put the mixture in a machine called a centrifuge, which spins it quickly.
- The heavier solids move to the bottom because of the spinning, and the liquid stays on top.
- We can then pour out the liquid to separate it from the solid.
- Example: We can separate butter from milk or blood cells from plasma using centrifugation.
Separating Liquid-Liquid Mixtures
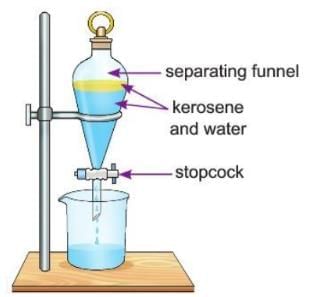
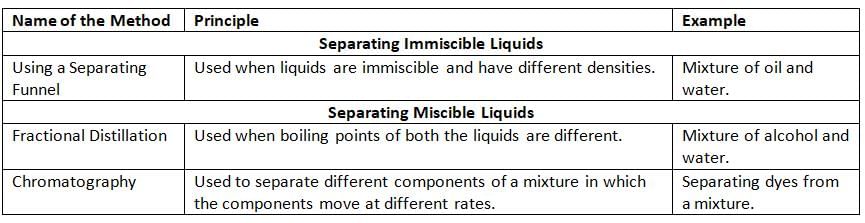
Using a Separating Funnel
- We use a separating funnel to separate liquids that don’t mix, like oil and water.
- A separating funnel is a glass tool with a tap at the bottom called a stopcock.
- We pour the mixture into the funnel and let it settle—the heavier liquid goes to the bottom, and the lighter liquid stays on top.
- We open the stopcock to let the heavier liquid (like water) come out first, then collect the lighter liquid (like oil) in another container.
Activity 3.4
Aim: To separate a mixture of kerosene and water.
Materials required: Mixture of kerosene and water, separating funnel.
Procedure:
- Take the mixture of kerosene and water in a separating funnel.
- Let the mixture sit for some time so the water settles down and the kerosene floats on top.
- Carefully open the stopcock and drain the water into a beaker.
Observation: The water settles down because it is heavier, and kerosene floats above it.
Conclusion: This shows that a mixture of kerosene and water can be separated using a separating funnel.
Fractional Distillation
- Fractional distillation is a way to separate two liquids that don’t mix by heating them.
- We use a special setup with a distillation flask, a fractionating column, a condenser, and a thermometer.
- We heat the mixture in the distillation flask.
- The liquid with the lower boiling point turns into vapor first and rises up the fractionating column.
- The vapor cools in the condenser and turns back into a liquid, which we collect.
- The liquid with the higher boiling point stays in the distillation flask.
- Example: We can separate alcohol and water using fractional distillation.
- Fractional distillation is used to separate things like crude oil into kerosene, gasoline, diesel, and paraffin wax.
- It is also used in oil refineries, chemical plants, and to separate pure gases.
Chromatography
- Chromatography is a way to separate different things in a mixture, like colors in ink.
- We use a special paper called chromatographic paper or filter paper.
- We draw a line near the bottom of the paper with a pencil.
- We put a drop of the mixture (like ink) on the line.
- We dip the paper in a liquid (called the solvent) like water or alcohol, but the line should not touch the liquid.
- The liquid moves up the paper and carries the mixture with it.
- Different parts of the mixture move at different speeds and make spots on the paper.
- We can see the different colors or parts of the mixture as spots on the paper.
- We take the paper out of the liquid and let it dry.
- The spots on the paper are called chromatograms.
Paper Chromatography
- Paper chromatography is a simple way to do chromatography using special paper called chromatographic paper.
- We take the paper and draw a line near the bottom with a pencil.
- We put a drop of the mixture (like ink) on the line and dip the paper in a liquid (like water or alcohol).
- The liquid moves up the paper and carries the colors in the ink with it.
- Different colors move at different speeds, so we see separate spots of color on the paper.
Advantages of Chromatography
- Chromatography can separate very small amounts of a substance.
- It can separate things that have very similar features.
- It helps us find out what’s in a mixture.
- It helps us measure how much of each thing is in a mixture.
- It helps us check if a substance is pure.
- It helps us separate harmful things, tiny germs, or bad stuff in food.
- Paper chromatography is used to separate colors in a mixture and check the quality of food, like finding vitamins, preservatives, amino acids, and proteins.
Did You Know?
- Thomas Graham (1805-1869): He worked on how gases spread and made a rule called Graham’s Law.
- Robert Boyle (1627-1691): He was one of the first people to use science methods in chemistry and physics.
List Some Common Methods of Separation of Mixtures
Methods to Separate Solid-Solid Mixtures: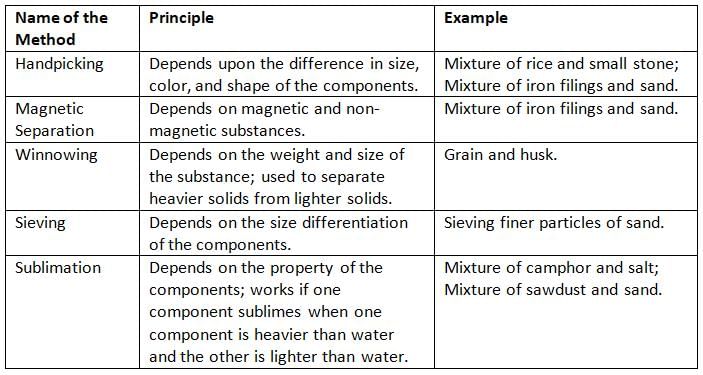
Methods to Separate Solid-Liquid Mixtures:
Methods to Separate Liquid-Liquid Mixtures:
Points To Remember
- Matter is anything that has weight and takes up space.
- Matter can be divided into two groups: pure substances and mixtures.
- Pure substances are either elements or compounds.
- A pure substance has a fixed amount of pieces, a definite melting and boiling point, and can be separated by simple methods.
- The pieces in a mixture keep their own features, can be separated by simple methods, and don’t have a fixed melting or boiling point.
- No energy is used or given off when making a mixture.
- Mixtures can be homogeneous or heterogeneous.
- Heterogeneous mixtures can be suspensions or emulsions.
- Mixtures can be in forms like solid-solid, solid-liquid, liquid-liquid, and more.
- We separate mixtures to get useful things, make pure substances, or remove harmful stuff.
- We use the differences in features of the pieces to separate them.
- We can use methods like handpicking, sieving, winnowing, magnetic separation, gravitation, solvent extraction, fractional crystallisation, sublimation, sedimentation, decantation, filtration, evaporation, distillation, crystallisation, centrifugation, fractional distillation, chromatography, and more to separate mixtures.
- We can use more than one method to separate mixtures with many pieces.
Glossary
- Matter: Anything that has weight and takes up space.
- Pure substance: A substance that has a fixed amount of pieces and fixed chemical features.
- Element: A substance that cannot be broken into simpler substances.
- Mixture: A mix of two or more substances like elements or compounds that keep their own features.
- Homogeneous mixture: A mixture where the pieces are mixed evenly all over.
- Heterogeneous mixture: A mixture where the pieces are not mixed evenly.
- Suspension: A mixture where one piece doesn’t fully mix and settles down.
- Emulsion: A mixture of two liquids that don’t mix—they form tiny drops in each other.
- Alloy: A mixture of a metal with another metal.
- Handpicking: A method where we pick pieces of a mixture with our hands.
- Sieving: A method where we use a sieve to separate pieces of a mixture based on size.
- Winnowing: A method of separating pieces of a mixture using wind or moving air.
- Magnetic separation: A method to separate a mixture where one piece is made of iron and gets attracted to a magnet.
- Gravitation: A method to separate a mixture when one piece is heavier than water and the other is lighter.
- Solvent extraction: A method to separate pieces of a mixture based on how well they dissolve in a liquid.
- Fractional crystallisation: A method to separate pieces of a mixture when their dissolving ability is different in the same liquid.
- Sedimentation: A method to let heavy pieces in a mixture settle down in a liquid.
- Sediments: The heavy pieces that settle down in sedimentation.
- Supernatant: The clear liquid above the sediments.
- Decantation: A method of pouring the clear liquid above the sediments without disturbing them.
- Filtration: A method to separate solid pieces from a liquid using a filter.
- Evaporation: A method where a liquid turns into a gas by heating or leaving it in the sun.
|
9 videos|37 docs|9 tests
|
FAQs on Elements, Compound and Mixtures Chapter Notes - Chemistry Class 8 ICSE
| 1. What is the difference between elements, compounds, and mixtures? |  |
| 2. How can mixtures be separated? |  |
| 3. What are some examples of mixtures and their components? |  |
| 4. What is a pure substance, and how is it different from a mixture? |  |
| 5. What methods can be combined for effective separation of mixtures? |  |