Hydrogen Chapter Notes | Chemistry for SSS 2 PDF Download
| Table of contents |

|
| Introduction |

|
| Occurrence of Hydrogen |

|
| Discovery of Hydrogen |

|
| Preparation of Hydrogen |

|
| Properties of Hydrogen |

|
| Oxidation and Reduction |

|
| Uses of Hydrogen |

|
| Points To Remember |

|
| Glossary |

|
Introduction
Hydrogen is a very special element in chemistry. It is the first element in the periodic table and the most common element in the universe. It is found in stars like the sun, where it helps produce energy, and on Earth, it is present in water, air, and many other things around us. In this chapter, we will learn how hydrogen is made, its properties, and how it is used. We will also study how scientists discovered hydrogen and the different ways it can be prepared in labs and industries.
Occurrence of Hydrogen
- Hydrogen is the first element in the periodic table and the most common element in the universe.
- It is found in large amounts in the sun and stars, which are mainly made of hydrogen and helium.
- Two hydrogen atoms join together to form a hydrogen molecule (H₂).
- Hydrogen makes up about 90% of the matter in the universe.
- In the sun and stars, hydrogen is a major part, but on Earth, it makes up only 1% of the air, water, and Earth's crust.
- Most hydrogen on Earth is found in water (H₂O), which covers 70% of the Earth's surface.
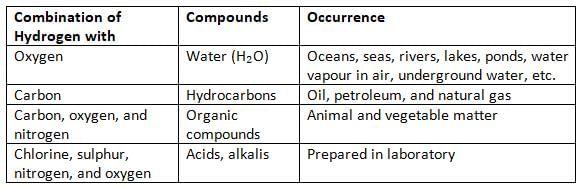
- Hydrogen also combines with other elements to form acids, bases, hydrocarbons, carbohydrates, proteins, and fats.
- On Earth, free hydrogen is found in small amounts in volcanic gases and clouds.
Compounds of Hydrogen on the Earth
Discovery of Hydrogen
- In 1767, Robert Boyle found that a gas came out when he mixed iron filings with dilute acids, and this gas burned easily.
- In 1766, Henry Cavendish called this gas "inflammable air" or "phlogiston" and said it was a new element.
- Henry Cavendish studied hydrogen's properties and found that when hydrogen burns, it makes water.
- In 1781, Antoine Lavoisier, a French scientist, named this gas "hydrogen," which means "water-forming" in Greek.
Did You Know?
- It is believed that a German-Swiss scientist named Paracelsus studied hydrogen in the 16th century. Paracelsus found that a flammable gas was made when a metal was dissolved in an acid.
- Henry Cavendish was an English chemist and physicist. He was born on October 10, 1731, in Nice, France. He is famous for discovering hydrogen as an element and for finding out that burning hydrogen makes water.
- Antoine Lavoisier was born on August 26, 1743, in Paris, France. He named the gas "hydrogen" and studied how oxygen helps in rusting metals and in animal and plant breathing.
Preparation of Hydrogen
- In labs and industries, hydrogen is made from compounds because free hydrogen is not easily available.
- Hydrogen is prepared by breaking down compounds like water, dilute acids, and alkalis using reactive metals.
- These reactions are called displacement reactions because hydrogen is pushed out of the compound.
Activity Series of Metals
- Reactive metals like sodium and calcium can easily push hydrogen out of acids or water.
- Less reactive metals like copper, silver, and mercury cannot push hydrogen out.
- Metals are arranged in order of their reactivity in the activity series of metals (Fig. 7.1).
Activity Series of Metals
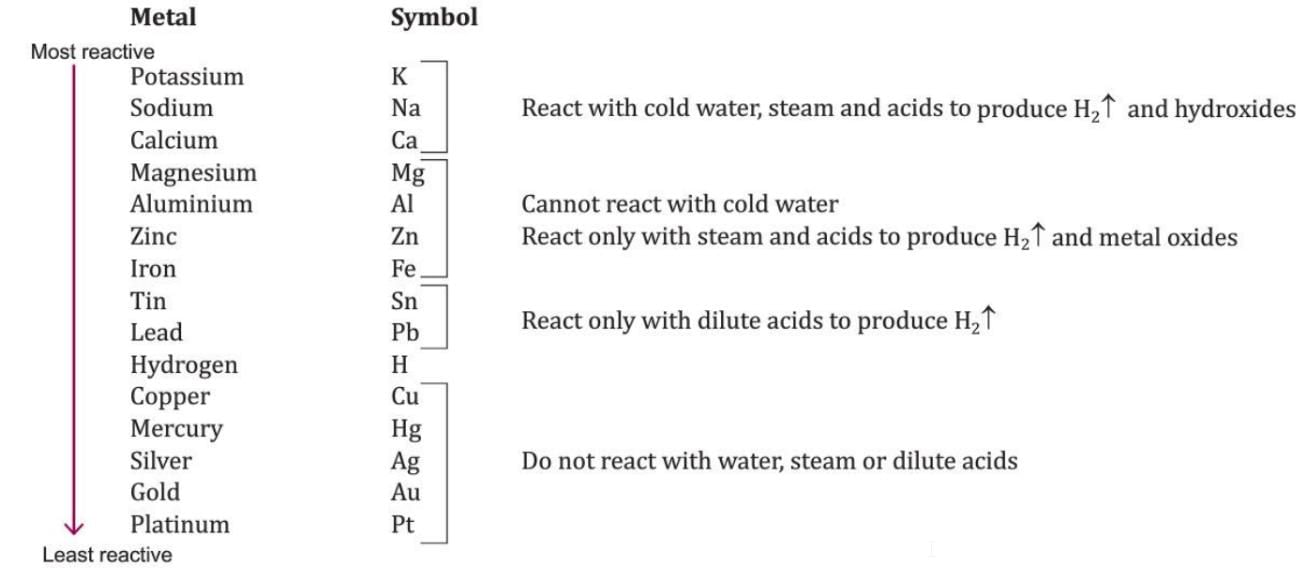
- The most reactive metal is at the top, and the least reactive metal is at the bottom of the series.
- Hydrogen is a non-metal but is included in this series for reference.
- Metals below hydrogen in the activity series do not push hydrogen out of water or dilute acids.
Preparation of Hydrogen by Action of Water on Metals
- From Cold Water: Metals like Potassium, Sodium, and Calcium
Metals like potassium, sodium, and calcium react with cold water to release hydrogen and form hydroxides.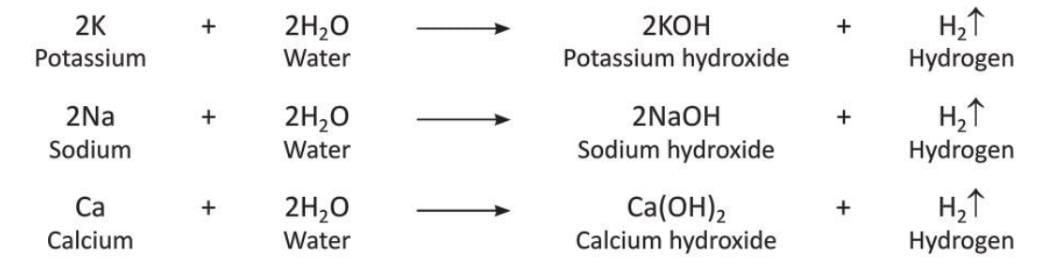 Potassium and sodium react so strongly with cold water that the hydrogen produced can catch fire immediately.
Potassium and sodium react so strongly with cold water that the hydrogen produced can catch fire immediately. - From Hot Water: Magnesium
Magnesium reacts with hot water to release hydrogen and form magnesium hydroxide.
- From Steam: Metals like Aluminium, Zinc, and Iron
Metals like aluminium, zinc, and iron react with steam to release hydrogen and form corresponding oxides.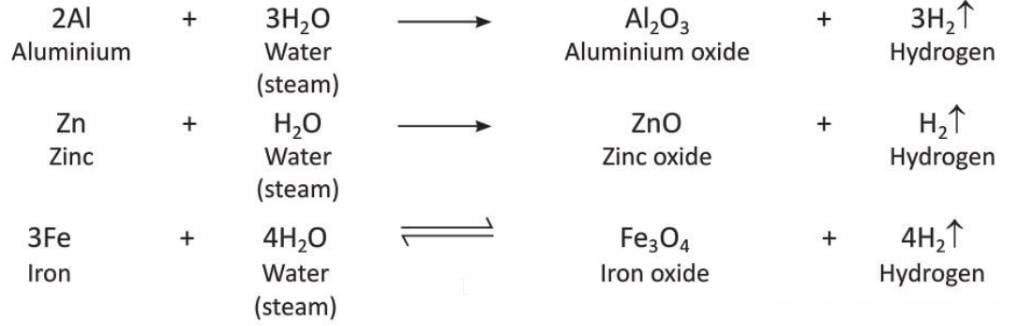
Preparation of Hydrogen by Action of Dilute Acids on Metals
Metals above hydrogen in the activity series react with dilute acids like sulphuric acid (H₂SO₄) or hydrochloric acid (HCl) to release hydrogen.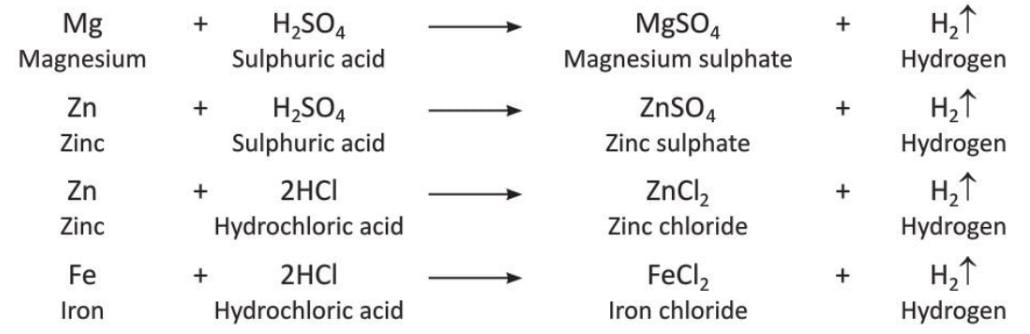
- Reactions with potassium, sodium, and calcium are not done because they are very explosive and dangerous.
- Reactions with lead stop because the product (PbCl₂ or PbSO₄) is insoluble and forms a layer over the metal, preventing further reaction.
- Dilute nitric acid (HNO₃) is not used to prepare hydrogen because it is a strong oxidising agent and produces nitric oxide or nitrogen dioxide instead.
Preparation of Hydrogen by Action of Alkalis on Metals
Metals like aluminium, zinc, and lead react with hot alkali solutions like sodium hydroxide to release hydrogen.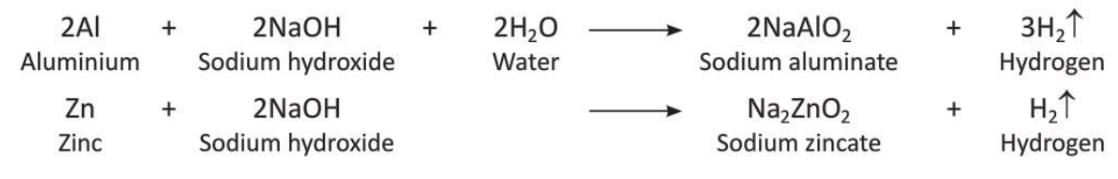

Preparation of Hydrogen by Electrolysis of Water
- When electricity is passed through acidulated water, it breaks down into hydrogen and oxygen.
- Acidulated water is made by adding a few drops of dilute sulphuric acid to pure water to make it a better conductor of electricity.
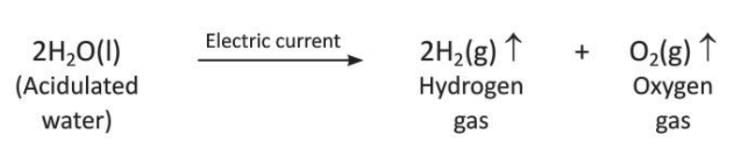
- Hydrogen is collected at the cathode (negative electrode), and oxygen is collected at the anode (positive electrode) in a 2 : 1 volume ratio.
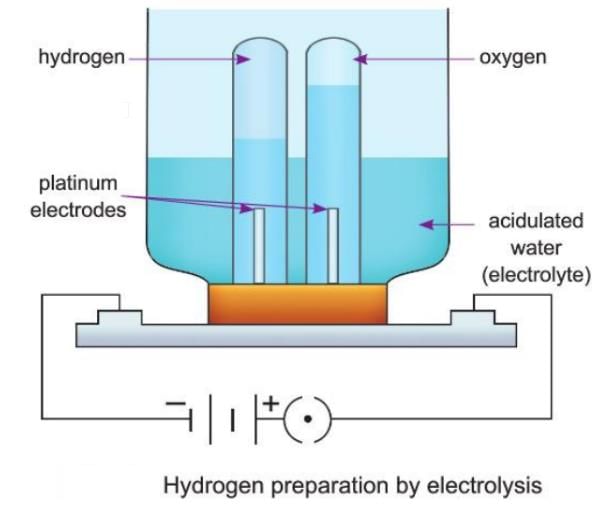
- Electrodes are rods made on inert (unreactive) materials like platinum to conduct electricity without reacting with the electrolyte.
- The device used for this process is called an electrolytic cell.
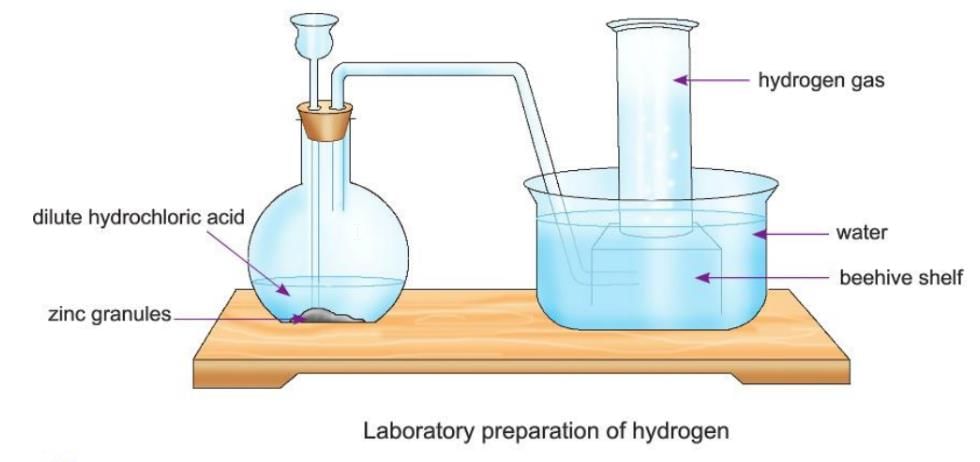
Laboratory Preparation of Hydrogen
- In the lab, hydrogen is prepared by reacting zinc granules with dilute sulphuric acid (H₂SO₄) or dilute hydrochloric acid (HCl).
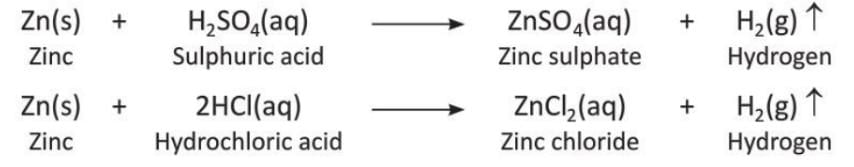
- Zinc granules are placed in a flat-bottom flask, and a thistle funnel is used to add the acid.
- The flask is closed with a two-holed rubber stopper; one hole has the thistle funnel, and the other has a delivery tube.
- The delivery tube is placed inside a beehive shelf in a trough of water, and a gas jar is inverted over the shelf to collect the gas.
- Dilute acid is poured through the thistle funnel until the zinc granules are covered, and hydrogen gas is produced.
- The gas bubbles rise up, pass through the delivery tube, and are collected in the gas jar by pushing the water down.
- The setup shows zinc granules in a flask with dilute acid, a thistle funnel, a delivery tube, a beehive shelf, and a gas jar to collect hydrogen.
Safety Precautions
- The setup must be airtight so that no gas leaks out, as hydrogen can cause an explosion.
- No flame should be near the setup because hydrogen is flammable and can catch fire if it leaks.
- The lower end of the thistle funnel should be completely covered with dilute acid to prevent gas leakage.
- The first few bubbles from the delivery tube should not be collected as they contain air.
Bosch's Process: Industrial Production of Hydrogen
Bosch's process is used to make hydrogen on a large scale in industries.
It has three main steps:
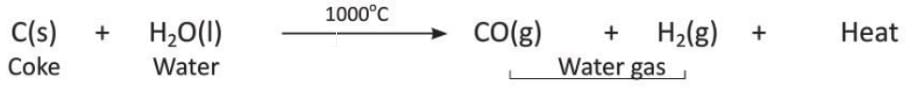
- Step 1: Production of Water Gas
- Water gas is a mixture of carbon monoxide (CO) and hydrogen (H₂).
- It is made by passing superheated steam over carbon (white-hot coke) at about 1000°C.
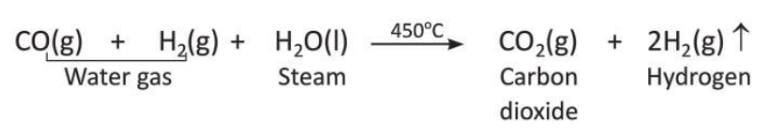
- Step 2: Conversion of Carbon Monoxide to Carbon Dioxide
- The mixture of CO and H₂ is mixed with steam and heated to 450°C in the presence of iron chromate and chromic oxide as catalysts.
- Carbon monoxide turns into carbon dioxide, and more hydrogen is produced.
- Step 3: Separation of Hydrogen from the Mixture
- The mixture is passed through water under 30 atmospheric pressure; carbon dioxide dissolves in water, and hydrogen is collected.
- The hydrogen is purified further using chemical methods.
- Carbon dioxide and carbon monoxide can be removed by passing them through KOH and ammoniacal CuCl, respectively.
Properties of Hydrogen
Physical Properties of Hydrogen
- Hydrogen is a colourless, odourless, tasteless, and non-poisonous gas.
- It is very light; 1 litre of hydrogen weighs only 0.09 g at 1 atmospheric pressure and 0°C.
- It is 20 times lighter than air.
- Hydrogen does not dissolve easily in water; 1 litre (1000 cm³) of water dissolves only 21.4 cm³ of hydrogen at ordinary temperature and pressure.
- Hydrogen can be turned into a liquid at a very low temperature of -240°C and 40 atm pressure.
- Its melting point is -259°C, and its boiling point is -253°C.
- Hydrogen is a highly flammable gas; it burns with a "pop" sound and has a pale blue flame.
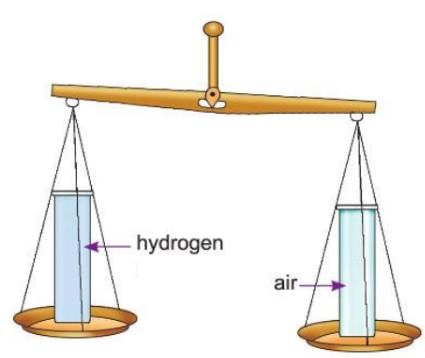
Activity 7.1
Aim: To show that hydrogen gas is lighter than air.
Materials required: Some hydrogen gas, a balance, two glass jars with lids.
Procedure:
- Take two glass jars of equal size and weight.
- One jar contains air; fill the other jar with an equal volume of hydrogen and place the lid on both jars.
- Place the two jars with their lids on the pans of a balance.
Observation: The jar containing hydrogen is lighter than the jar containing air.
Conclusion: Hydrogen gas is lighter than air.
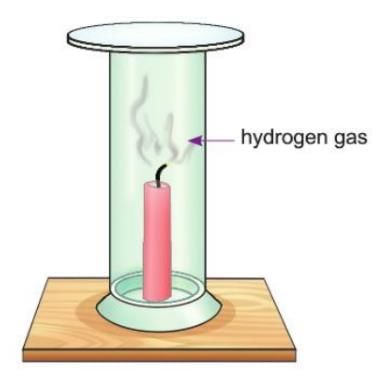
Activity 7.2
Aim: To show that hydrogen gas is combustible.
Materials required: A glass jar filled with hydrogen, a lighted candle.
Procedure:
- Take a gas jar filled with hydrogen.
- Invert it over a burning candle as shown in the figure.
Observation: The candle goes out, and the gas burns with a "pop" sound.
Conclusion: This shows that hydrogen gas is combustible.
Chemical Properties of Hydrogen
- Action with Litmus: Hydrogen is a neutral gas; it does not change the colour of litmus paper (neither acidic nor basic).
- Combustibility: Hydrogen burns in air with a pale blue flame, producing water vapour and releasing heat energy.
- Reaction with Oxygen: Hydrogen burns in oxygen with a pale blue flame to form water; this reaction releases a lot of energy and is used to propel space rockets.
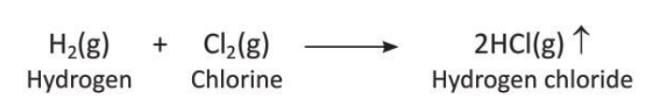
- Reaction with Chlorine: Hydrogen and chlorine combine slowly in diffused sunlight and explosively in bright sunlight to form hydrogen chloride gas.
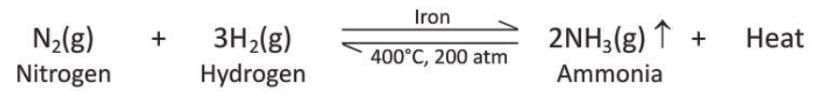
- Reaction with Nitrogen: Hydrogen does not react with nitrogen under normal conditions. In the presence of finely divided iron dust as a catalyst, at 450°C and 200 atm pressure, hydrogen reacts with nitrogen to form ammonia (this is called the Haber’s process). The reaction produces ammonia in a 3 : 1 volume ratio and is reversible; it is exothermic (releases heat).
- Reaction with Sulphur: When hydrogen gas is passed through molten sulphur, it forms hydrogen sulphide gas, which smells like rotten eggs.

- Reaction with Carbon Dioxide: At 300–400°C under high pressure with nickel as a catalyst, hydrogen reacts with carbon dioxide to form methane and water. This reaction, called the Sabatier reaction, is used to produce water for astronauts in the International Space Station.

- Reaction with Carbon: Hydrogen reacts with carbon at a temperature above 520°C with nickel or cobalt as a catalyst to form hydrocarbons like methane. The reaction can be reversed by raising the temperature further.

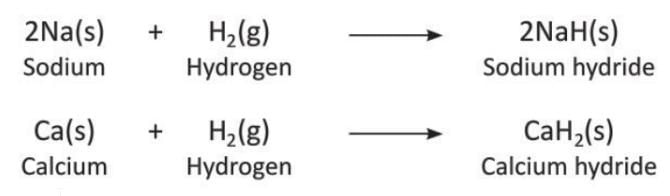
- Reaction with Metals: Hydrogen reacts with highly reactive metals at high temperatures to form salt-like compounds called metal hydrides.
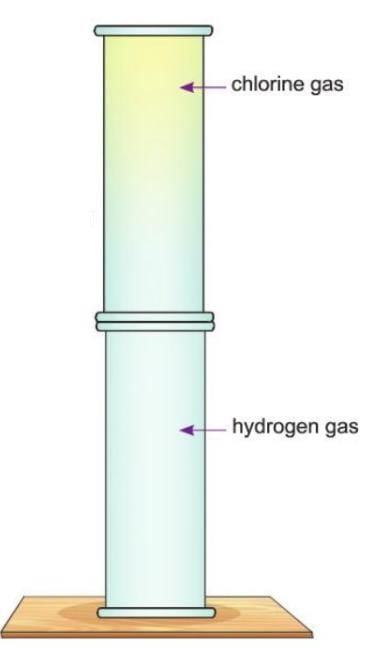
Activity 7.3
Aim: To show the formation of hydrogen chloride gas.
Materials required: A jar filled with hydrogen gas, a jar filled with chlorine gas.
Procedure:
- Take two jars; fill one jar with hydrogen gas and the other with chlorine gas.
- Invert the jar filled with chlorine over the jar filled with hydrogen gas.
- Remove the lids of both jars to mix the two gases.
- Expose the two jars to sunlight.
- Now bring a glass rod dipped in ammonia solution near the mouth of the gas jar.
Observation: The greenish-yellow colour of chlorine gas disappears, and dense white fumes of ammonium chloride are produced.
Conclusion: This shows the formation of hydrogen chloride gas when hydrogen reacts with chlorine in the presence of diffused sunlight.
Did You Know?
The mixture of hydrogen and oxygen, when lit by a spark, causes an explosion.
Tests for Hydrogen
- Test 1: Pure hydrogen burns with a pale blue flame in air, but impure hydrogen burns in air with a "pop" sound. When a few drops of water are sprinkled on white anhydrous copper sulphate, it turns into blue anhydrous copper sulphate, showing that water is formed during burning.
- Test 2: When hydrogen is burned in air, water is formed. If a few drops of this water are sprinkled on white anhydrous copper sulphate, it turns into blue anhydrous copper sulphate, confirming the presence of water.
Oxidation and Reduction
- Oxidation: Adding oxygen or removing hydrogen from a substance is called oxidation.
- Reduction: Adding hydrogen or removing oxygen from a substance is called reduction.
- Oxidation is when oxygen is added to an element or compound.
- Reduction is when oxygen is removed or hydrogen is added to an element or compound.
- A substance that causes oxidation is called an oxidizing agent.
- A substance that causes reduction is called a reducing agent.
- In some reactions, both oxidation and reduction happen at the same time. This is called a redox reaction.
- In a redox reaction, metals like copper, lead, iron, and zinc lose oxygen when hydrogen gas is passed over their hot oxides. Hydrogen itself gets oxidized into water.
- Here are some examples of redox reactions with hydrogen:
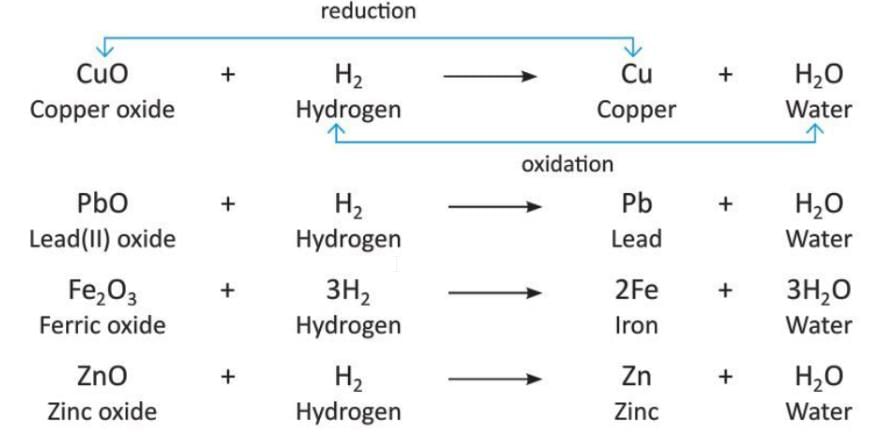
- In these reactions, hydrogen acts as a reducing agent because it removes oxygen from the metal oxides.
Did You Know?
The reaction between iron and steam is a reversible reaction.
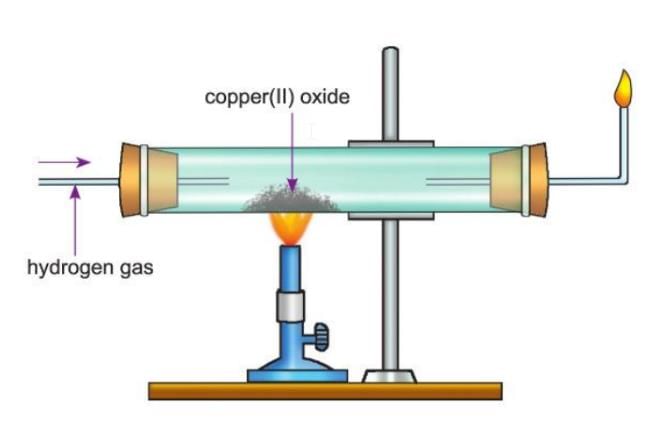
Activity 7.4
Aim: To show that hydrogen gas is a strong reducing agent.
Materials Required:
- A hard glass combustion tube.
- Black copper(II) oxide.
- A burner.
- A bent glass tube.
- A glass tube.
- Two corks.
Procedure:
- Take a hard glass combustion tube.
- Put some black copper(II) oxide inside the tube.
- Set up the apparatus as shown in the figure.
- Pass hydrogen gas over the heated copper(II) oxide.
- Light the hydrogen gas coming out from the other end.
- Stop heating but keep passing hydrogen gas until the tube cools down.
- This is done to prevent the hot copper from mixing with oxygen in the air and forming copper(II) oxide again.
Observation:
- The black copper(II) oxide changes into reddish-brown copper metal.
- The reaction is: Copper(II) oxide (black) + Hydrogen → Copper (reddish-brown) + Water.
Conclusion: This shows that hydrogen is a strong reducing agent because it changes copper(II) oxide into copper by removing oxygen.
Uses of Hydrogen
- Hydrogen is used a lot to make ammonia (NH₃) by a method called Haber’s process.
- Ammonia is used to make fertilizers and nitric acid.
- Hydrogen is used to make hydrochloric acid and methanol.
- Hydrogen is used to make solid vanaspati ghee from liquid vegetable oils like palm oil, groundnut oil, and coconut oil.
- In this process, liquid vegetable oils are treated with hydrogen under pressure, with finely divided nickel, platinum, or palladium as a catalyst. This is called catalytic hydrogenation of oils.
- Hydrogen is used to make an oxy-hydrogen flame, which is very hot and used for cutting and welding metals.
- The oxy-hydrogen flame has a temperature of about 2800°C.
- Liquid hydrogen is used as a fuel in rockets and guided missiles.
- This is because hydrogen gives a lot of heat when it burns, even more than coal gas and water gas.
- Hydrogen is used as a reducing agent in metallurgy to get pure metals from their oxides.
- Hydrogen is seen as a future source of energy because it does not cause pollution and is a clean fuel.
- It can replace harmful gases like hydrocarbons in burning.
Coal Gas
- Coal gas is a gaseous mixture made of hydrogen, methane, and carbon monoxide.
- It is formed by the destructive distillation of coal.
Water Gas
- Water gas is a gaseous mixture made of carbon monoxide and hydrogen.
- It is formed by passing steam over red-hot coke.
Metallurgy
- Metallurgy is a science that studies the properties of metals and how to separate them from their ores.
Points To Remember
- Hydrogen is the simplest and the lightest of all elements.
- Hydrogen makes up almost 90% of the matter in the universe.
- Hydrogen makes up only 1% of the Earth’s crust, oceans, and atmosphere.
- An atom of hydrogen consists of one proton around which one electron revolves.
- Hydrogen combines with other elements to form water, acids, bases, hydrocarbons, carbohydrates, proteins, and fats.
- In 1766, Henry Cavendish discovered the element hydrogen.
- Henry Cavendish discovered hydrogen from water, acids, and alkalis by metals placed above it in the activity series.
- Hydrogen is prepared by the action of water on metals, dilute acids on metals, alkalis on metals, and by the electrolysis of water.
- In the laboratory, hydrogen is prepared by the action of dilute sulphuric acid or dilute hydrochloric acid on zinc granules.
- Hydrogen is combustible in air but it does not support combustion.
- Pure hydrogen burns with a pale blue flame in air.
- Impure hydrogen burns in air with a characteristic pop sound.
- Hydrogen is used in the production of ammonia, hydrochloric acid, methanol, ammonia, etc., and for other purposes.
- Hydrogen is seen as a future source of energy.
Glossary
- Displacement reactions: Reactions in which hydrogen gets displaced from its compounds like water, acids, and alkalis by more reactive metals.
- Activity series of metals: A list of metals arranged in order of their decreasing reactivity.
- Electrolysis: A process in which acidulated water dissociates into hydrogen and oxygen on the passage of electric current.
- Catalytic hydrogenation of oils: A process in which liquid vegetable oils are treated with hydrogen under pressure in the presence of finely divided nickel, palladium, or platinum to make solid vanaspati ghee.
- Oxidation: A process of adding oxygen to an element or compound.
- Reduction: A process of removing oxygen by adding hydrogen to an element or compound.
- Water gas: A mixture of carbon monoxide and hydrogen.
- Hydrocarbons: Compounds formed when hydrogen combines with carbon.
- Redox reaction: A reaction in which both oxidation and reduction occur simultaneously.
|
1 videos|45 docs|16 tests
|
FAQs on Hydrogen Chapter Notes - Chemistry for SSS 2
| 1. What is the occurrence of hydrogen in nature? |  |
| 2. Who discovered hydrogen and when? |  |
| 3. How is hydrogen prepared in the laboratory? |  |
| 4. What are the main properties of hydrogen? |  |
| 5. What are the uses of hydrogen in everyday life? |  |