Carbon and Its Compounds Chapter Notes | Chemistry Class 8 ICSE PDF Download
| Table of contents |

|
| Introduction |

|
| Occurrence of Carbon |

|
| Compounds of Carbon |

|
| Chemical Properties of Carbon |

|
| Allotropes of Carbon |

|
| Carbon Dioxide |

|
| Carbon Monoxide |

|
| Points To Remember |

|
| Glossary |

|
Introduction
Carbon is a very special element found in many things around us, like pencils, charcoal, and even diamonds! In this chapter, we will learn about carbon and its different forms, such as graphite, charcoal, and fullerenes. We will also study how these forms are made, their properties, and their uses in our daily life. There are fun activities to help us understand how carbon compounds work, like conducting electricity or removing colors from solutions. Let’s explore the amazing world of carbon!
Occurrence of Carbon
- Carbon is the 4th most common element in the universe.
- In nature, carbon is found in both free and combined forms.
- In its free form, carbon is found as diamond, graphite, and fullerene (a form discovered recently).
- In its combined form, carbon is found in compounds like:
- Carbon dioxide, which is a gas in the air.
- Carbonates like chalk, limestone (CaCO₃), marble, dolomite (CaCO₃·MgCO₃), and calcite (ZnCO₃).
- Fossil fuels like coal, petroleum, and natural gas.
- Organic compounds like carbohydrates, fats, and proteins.
- Clothing materials like cotton, wool, and silk.
- The Earth's crust has a very small amount of carbon, mostly in coal and petroleum.
- The air has only 0.03% carbon dioxide gas.
Compounds of Carbon
- There are more than 5 million carbon compounds known, and new ones are made in labs every day.
- The number of carbon compounds is much more than the compounds of all other elements combined.
- Carbon compounds are divided into two types: organic and inorganic.
- Organic compounds come from living things and include hydrocarbons and their derivatives.
- Inorganic compounds do not come from living things and include carbon dioxide, carbonates, and carbides.
- The study of organic compounds, like hydrocarbons, is called organic chemistry.
Organic and Inorganic Compounds
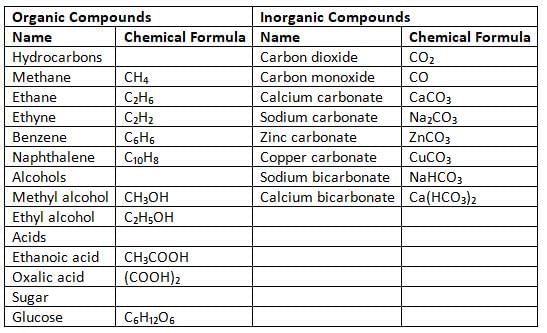
Carbon forms a large number of compounds because of its valency. It has an atomic number of 6 and an electronic configuration of (2, 4). With a valency of 4, carbon can create covalent bonds with many other carbon atoms and also with elements like hydrogen, oxygen, nitrogen, sulphur, and chlorine.
Some Useful Carbon Products
- Polyvinyl chloride (PVC) is used to make pipes.
- Bakelite is a strong plastic that does not change shape; it is used to make electrical switches, handles of utensils, telephones, and cameras.
- Polythene is a very common carbon product; it is used to make carry bags, pipes, and household items.
Fun Fact: Covalent Bond
A bond formed by sharing electrons between atoms is called a covalent bond.
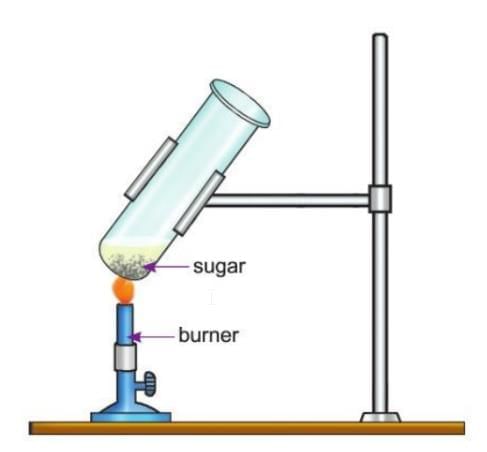
Activity 9.1
Aim: To show that sugar contains carbon.
Materials Required: Some sugar, a test tube, a burner.
Procedure:
- Take some sugar in a test tube.
- Heat the test tube over a burner for some time.
Observation: The sugar melts and turns brown. It finally becomes charred and turns black.
Conclusion: The black substance left after heating is carbon. This shows that sugar has carbon in it.
When you burn other things like coal, wood, or paper, a black residue of carbon is also left behind.
Chemical Properties of Carbon
- Carbon does not dissolve in any solvents.
- Carbon likes oxygen a lot; when heated with a lot of oxygen, it forms carbon dioxide.

- But if carbon is heated with a limited amount of oxygen, it forms carbon monoxide.
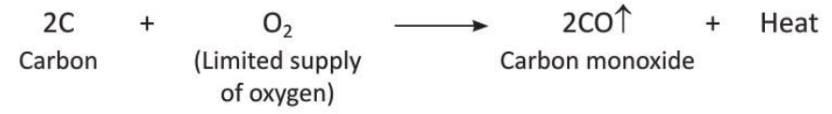
- But if carbon is heated with a limited amount of oxygen, it forms carbon monoxide.
- At 500°C and 250 atmospheric pressure, carbon reacts with hydrogen to form hydrocarbons like methane and ethane.
- Carbon is a good reducing agent; it removes oxygen from metal oxides (listed in the metal activity series) to form metals.

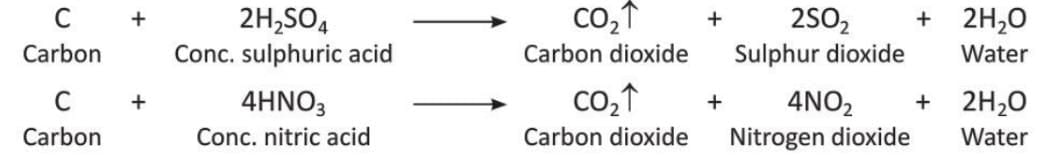
- Carbon reacts with concentrated sulphuric acid and nitric acid to form carbon dioxide, sulphur dioxide, nitrogen dioxide, and water.
- Carbon in the form of wood charcoal reacts with sulphur vapours when heated strongly to form carbon disulphide.
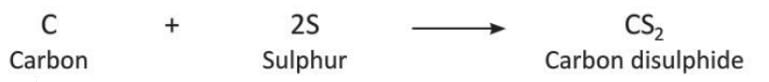
- Carbon reacts with calcium at a very high temperature (1000°C to 2800°C) to form calcium carbide.

- When steam is passed over red-hot coke, it forms a mixture of carbon monoxide and hydrogen called water gas.
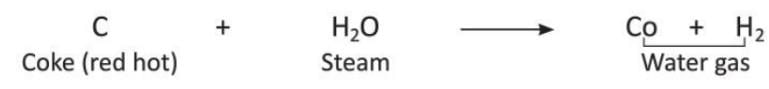
- Carbon in the form of finely ground coke, when heated strongly with an electric arc, reduces silicon dioxide to silicon carbide.

Allotropes of Carbon
- Some elements can exist in different physical forms but have the same chemical properties; this is called allotropy.
- The different forms of an element are called allotropes.
- Carbon has two types of allotropes: crystalline and amorphous.
- Crystalline forms of carbon are diamond, graphite, and fullerene.
- Amorphous forms of carbon are charcoal (like wood charcoal, bone charcoal, and sugar charcoal), coke, gas carbon, lampblack, and coal.
- All forms of carbon have different physical properties but produce carbon dioxide and release heat when burned in oxygen.
Crystalline Forms of Carbon
Diamond
- Diamond is the purest form of carbon.
- It is the hardest natural substance known; it can only be cut by another diamond.
- Diamonds come in different colours like grey, brown, black, and pink due to impurities.
- A pure diamond is colourless.
Occurrence of Diamond
- Diamonds are found in many places like South Africa, Brazil, Namibia, the Democratic Republic of Congo, Botswana, Belgium, Russia, Australia, the USA, and India.
- In India, diamonds are found in Golconda (Karnataka) and Panna (Madhya Pradesh).
- About 70% of the world’s demand for diamonds comes from these places.
- Diamond-bearing rocks are called kimberlite rocks, named after the town of Kimberley in South Africa.
Natural Diamonds
- Natural diamonds form when carbon is trapped in molten lava under high pressure and temperature.
- They are found deep in the Earth at about 150 km, brought up by volcanic eruptions in kimberlite rocks.
Artificial or Synthetic Diamonds
- Scientists can make artificial diamonds by heating pure carbon to about 3000°C under very high pressure.
- These artificial diamonds are used for cutting and grinding tools.
Structure of Diamond
- In a diamond, each carbon atom is linked to four other carbon atoms with covalent bonds.
- The four carbon atoms around each carbon form the corners of a tetrahedron.
- The carbon atoms are packed tightly in a three-dimensional rigid structure, making diamond very hard.
Diamond Value
- A diamond's value is based on its weight and how pure it is. Diamond weight is measured in carats, where 1 carat equals 200 milligrams or 0.2 grams. The well-known Kohinoor diamond had a weight of 186 carats.
- The quality of a diamond is usually described using the four C’s: carat (weight), cut, colour, and clarity. Diamonds that are clear, colourless, and transparent are the most valuable because they have very few impurities.
Properties of Diamond
- Diamond is a colourless, transparent substance with great shine after cutting and polishing.
- Raw diamonds do not shine; they shine after cutting and polishing due to the reflection of light.
- The different colours in diamonds are due to small amounts of metal oxides and salts.
- Diamond is the hardest natural substance with a density of 3.5 g/cm³.
- Diamond does not conduct electricity but conducts heat very well.
- Diamond has a high melting point of 3500°C.
- When heated to 800°C in the presence of oxygen, diamond forms carbon dioxide gas.
- Diamond does not dissolve in any solvents.
- Diamond does not react with any chemicals.
- The value of a diamond depends on its weight and impurities; 1 carat = 200 mg = 0.2 g.
- The famous Kohinoor diamond weighs 186 carats.
- The four Cs—carat, cut, colour, and clarity—determine the quality of a diamond.
- Colourless, transparent diamonds are the most expensive as they have very few impurities.
- Black diamonds are the rarest of all.
- Diamond is transparent to visible light and X-rays; this helps tell a real diamond from a fake one.
Uses of Diamond
- Diamonds are used in tools like glass cutters and rock drilling equipment.
- Diamonds are used to make jewellery because of their great shine.
- Diamonds are used by eye surgeons as a precise tool for eye surgeries.
- Diamond dust is used for polishing other diamonds and valuable stones.
- Diamonds can withstand high radiation in space and are used to make protective windows for spacecraft and satellites.
Fun Fact
- The Earth’s crust has only a small amount of carbon, mostly in coal and petroleum. The atmosphere has only 0.03% of carbon dioxide gas.
- The study of organic compounds like hydrocarbons is called organic chemistry.
- Diamond-bearing rocks are called kimberlite rocks, named after the town of Kimberley in South Africa.
- India was once famous for the ‘Kohinoor’ diamond found in Wajrakarur (Andhra Pradesh); it now adorns the crown of the British queen.
- Black diamonds are the rarest of all.
- Diamond is transparent to visible light and X-rays; this helps tell a real diamond from a fake one.
- Ferdinand Frederick Henri Moissan (1852–1907) was the first person to make artificial diamonds. In 1892, he made artificial diamonds in an electric-arc furnace. In the furnace, he heated a mixture of sugar charcoal and iron to about 4000°C. He proposed that diamond could be made by crystallization of carbon under pressure using molten iron.
Graphite
- Graphite is a greyish-black solid that is not see-through.
- Its name comes from a Greek word "graphein," which means "to write."
Occurrence of Graphite
- Graphite is found in nature in places like Sri Lanka and Siberia.
- It is also found in Russia, New Zealand, and the Southern Hemisphere.
- In some countries like the United States, India, and California, graphite is made artificially.
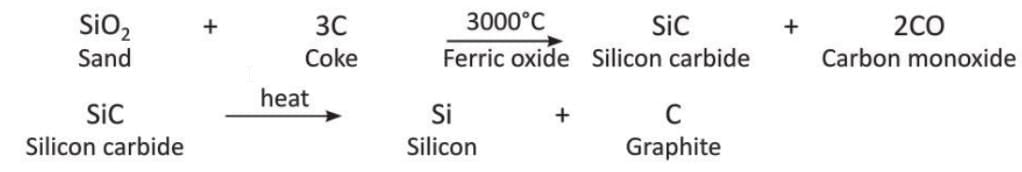
Preparation of Graphite
- Graph _ ite is made by heating a mixture of sand (SiO₂) and coke (3C) in an electric furnace at a very high temperature of 3000°C.
- During this process, a little sand and iron(III) oxide are added to the mixture.
Structure of Graphite
- Graphite is made of layers of carbon atoms.
- Each carbon atom in a layer is connected to three other carbon atoms by strong bonds, forming flat hexagonal rings.
- The layers are held together by weak forces, so they can slide over each other easily.
- This sliding property makes graphite soft.
- In each layer, a carbon atom is bonded to only three other carbon atoms using covalent bonds.
- Each carbon atom has four valence electrons, but only three are used in bonding, leaving one free electron.
- These free electrons in graphite help it conduct electricity.
Properties of Graphite
- Graphite is a greyish-black solid with a metallic shine.
- It feels soft and slippery when you touch it.
- Graphite is a good conductor of heat and electricity.
- Its density is between 1.9 to 2.3 g/cm³.
- Graphite has a very high melting point of 3700°C.
- It leaves a greyish-black mark on paper, which is why it is used in pencils.
- Graphite burns in air at 700°C to form carbon dioxide gas.
- It does not dissolve in most liquids and can be turned into a diamond at very high temperature and pressure.
Fun Fact
- The lead in our pencils is made by mixing graphite with clay. Since graphite does not vaporize, it can be used in machines that work at very high temperatures, where normal oils cannot be used.
- Graphite can be used as a dry lubricant in the form of powder or mixed with petroleum jelly to make graphite grease.
Uses of Graphite
- Graphite is soft, so its powder is used as a lubricant in fast-moving machine parts.
- It conducts electricity, so it is used to make electrodes in dry cells and electric arcs.
- Graphite is used to make pencil lead.
- It is black and does not dissolve in water, so it is used to make black paint and printer’s ink.
- Graphite is used in nuclear reactors to slow down fast-moving particles because it is a moderator.
- It is used to make crucibles for melting metals because it has a high melting point.
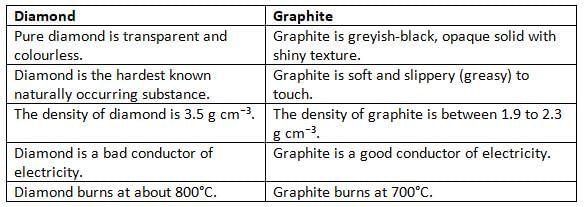
Differences between Diamond and Graphite
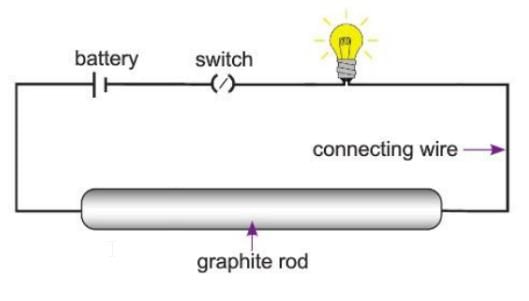
Activity 9.2
Aim: To show that graphite is a good conductor of electricity.
Materials required: A graphite rod, a battery, a bulb, a switch, connecting wires.
Procedure:
- Connect the graphite rod to a battery, bulb, and switch using connecting wires as shown in the diagram.
- Close the circuit by turning on the switch.
Observation: The bulb glows.
Conclusion: This shows that graphite can conduct electricity.
Fullerenes (Buckminsterfullerene)
- Fullerene is a form of carbon with 60 carbon atoms.
- It was discovered in 1985 by three scientists named Harry Kroto, Robert Curl, and Richard Smalley.
- Fullerene has a spherical, crystalline structure.
- It is also called buckminsterfullerene.
Properties of Buckminsterfullerene
- Buckminsterfullerene is a dark solid at room temperature.
- It is different from diamond and graphite, which are also forms of carbon.
- In diamond and graphite, atoms are tightly packed, but in buckminsterfullerene, atoms are loosely packed, making it a small molecule with only 60 carbon atoms.
- When heated, buckminsterfullerene turns into carbon dioxide gas, but diamond and graphite do not.
- Buckminsterfullerene is neither very hard nor very soft.
- It can dissolve in organic solvents.
- It conducts electricity poorly, with a specific gravity range of 1.8 to 2.1.
Uses of Buckminsterfullerene
- Some buckminsterfullerenes can act as superconductors, which means they can conduct electricity very well under special conditions.
- Since it is a bad conductor of electricity under normal conditions, it is used as an insulator (something that does not allow electricity to pass).
- It is used in flat-screen televisions.
- It is also used in the treatment of AIDS.
Amorphous Forms of Carbon
- Carbon can be found in different shapes and forms.
- Some forms of carbon do not have a proper shape or structure; they are called amorphous forms.
- Examples of amorphous forms are charcoal, coke, and carbon black.
- These forms break easily into smaller pieces and the surface area increases when broken.
Charcoal
- Charcoal is a black, porous, and brittle solid.
- It is made by heating wood in the absence of air.
- This process of heating wood without air is called destructive distillation.
- Big molecules break down into smaller ones during destructive distillation.
- When substances with low boiling points are heated, they escape, leaving behind charcoal.
- There are three types of charcoal: wood charcoal, bone charcoal, and sugar charcoal.
Wood Charcoal
- Wood charcoal is black, porous, and brittle.
- It is made by heating wood in the absence of air.
- It is a mixture of carbon (C), hydrogen (H), and oxygen (O).
- When heated for a long time, it becomes pure carbon (C).
- Wood charcoal is also called activated charcoal.
Physical Properties of Wood Charcoal
- Wood charcoal has a density of 1.5 g/cm³, which is more than water.
- It floats on water even though it is denser because it has air trapped in its pores.
- It is a bad conductor of heat and electricity.
- It is a good absorbent of gases, liquids, and solids.
- It can hold gases or liquids on its surface because of its ability to attract molecules.
Uses of Wood Charcoal
Wood charcoal is used as a fuel because:
- It catches fire at a lower temperature than wood.
- It has a higher energy value than wood.
- It produces less smoke, so it causes less air pollution than wood.
- Charcoal is used to reduce metals from their oxides during metal extraction.
- Since it can absorb things well, it is used in gas masks for military and industrial purposes.
- Charcoal is a part of gunpowder, which is an explosive used in guns and rifles.
- It can absorb colored materials, so it is used to remove colors from sugar solutions.
- Charcoal is used to make tablets that help with stomach problems like indigestion and gas.
- It is used to make deodorants because it can absorb bad-smelling gases.
- Charcoal is used in filters and sieves to clean air or water.
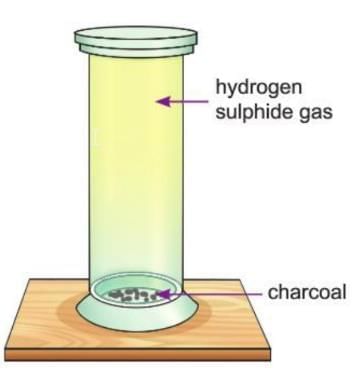
Activity 9.3
Aim: To show that wood charcoal is a good absorbent.
Materials required: A gas jar, some hydrogen sulphide gas, some wood charcoal.
Procedure:
- Take a gas jar and fill it with hydrogen sulphide gas, which has a strong, bad smell.
- Put some charcoal pieces in the jar and close the lid.
- Shake the jar and leave it still for some time.
- Remove the lid and smell it.
Observation: The bad smell of hydrogen sulphide gas is no longer there.
Conclusion: This shows that charcoal can absorb the gas and remove its smell.
Activated Charcoal
- Activated charcoal is made by heating wood charcoal at around 900°C with a limited supply of air.
- In powdered form, activated charcoal has more surface area than normal wood charcoal, so it can absorb more things effectively.
- Activated charcoal is used as a catalyst in many chemical reactions.
- It is also used to separate a mixture of noble gases.
Bone or Animal Charcoal
- Bone or animal charcoal is a black, granular substance.
- It contains only 10% carbon, along with a high amount of calcium phosphate and carbonate.
- It is made by boiling bones in water to remove fatty substances and then heating them to destroy organic matter.
- Bone charcoal is a good agent for removing colors from solutions.
Uses of Bone or Animal Charcoal
- It is widely used to remove color from cane sugar solutions.
- It is used to remove fluoride from water.
- It is used to make a large number of phosphorus compounds.
- Calcium phosphate in bone charcoal dissolves in hydrochloric acid, and the solution is filtered and heated to get black or ivory black, which is used as a pigment in artistic painting because it is a deep black color.
- It is used to filter aquarium water.
- It is used to refine crude oil to make petroleum jelly.
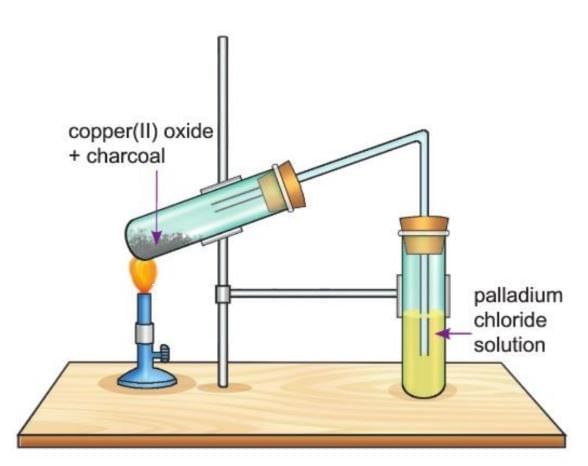
Activity 9.4
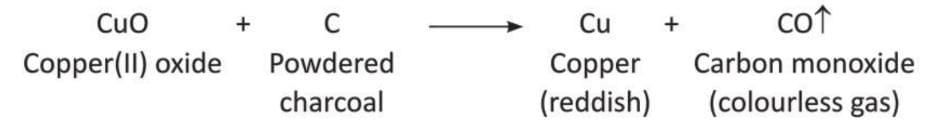
Aim: To show that charcoal is a good reducing agent.
Materials required: Two test tubes, some black copper(II) oxide, some powdered charcoal, some palladium chloride solution, a burner, a delivery tube.
Procedure:
- Set up the apparatus as shown in the diagram.
- Take some black copper(II) oxide in a test tube.
- Heat it with powdered charcoal.
- Pass the gas produced through the palladium chloride solution.
Observation: The palladium chloride solution turns pink, and a colorless gas is released. Reddish particles of copper metal are formed in the test tube.
Conclusion: This shows that charcoal (which has carbon) reduces copper(II) oxide to copper metal, and carbon monoxide gas is produced.
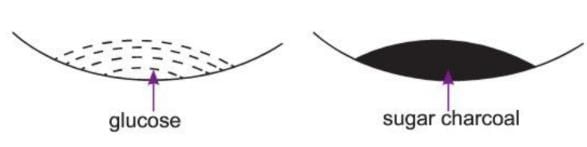
Sugar Charcoal
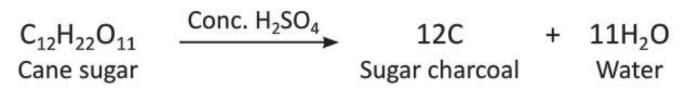
- Sugar charcoal is the purest form of carbon. It is made by heating cane sugar or glucose without air (destructive distillation).

- It can also be made by dehydrating cane sugar or glucose with concentrated sulphuric acid. In this process, a black mass is formed, which is washed and dried to get sugar charcoal.
Uses of Sugar Charcoal
- It is used as a reducing agent to extract metals from their oxides.
- It is used to remove color from solutions (decolourising agent).
- It is used to make artificial diamonds by heating it at 3000°C to 3500°C.

Activity 9.5
Aim: To observe that charcoal is a decolourising agent, i.e., it absorbs coloring matter.
Materials required: A beaker, some water, blue ink, some powdered charcoal.
Procedure:
- Take some water in a beaker and add a few drops of blue ink to it.
- Add some powdered charcoal to the beaker and stir well for some time.
Observation: The blue color of the solution fades away.
Conclusion: This shows that charcoal can absorb the color and act as a decolourising agent.
Activity 9.6
Aim: To show the formation of sugar charcoal.
Materials required: A watch glass, some glucose, some concentrated sulphuric acid.
Procedure:
- Take some glucose in a watch glass.
- Add a few drops of concentrated sulphuric acid to it.
Observation: The white glucose turns into a black, spongy mass of carbon.
Conclusion: The black mass is sugar charcoal.
Coke
- Coke is a greyish-black, solid material that burns with almost no smoke.
- It is made by the destructive distillation of coal and has about 95–98% carbon.
Types of Coke
- Coke is of two types:
- Hard coke: It is a light, lustrous (shiny) material used in industrial furnaces.
- Soft coke: It is a black, porous material used in household furnaces.
- When coal is heated without air during destructive distillation, it produces many by-products like coal gas, coal tar, and carbon gas.
- Coke is also a good reducing agent (it helps remove oxygen from compounds).
Uses of Coke
- It is a very important fuel for both homes and industries.
- It is used to get metals like zinc and iron from their ores by acting as a reducing agent.
- It is used to make water gas (a mix of CO and H₂), producer gas (a mix of CO and N₂), and artificial graphite.
- It is used to make metallic carbides, like calcium carbide, which is used in many industries.
Gas Carbon
- Gas carbon is made by heating hydrocarbons (like oil) at a very high temperature in a closed vessel.
- During this process, tiny carbon particles stick to the walls of the container and are collected to form gas carbon.
Uses of Gas Carbon
- Gas carbon is a good conductor of electricity, so it is used to make electrodes for dry cells and arc lamps.
Lampblack or Carbon Black or Soot
- Lampblack has 98–99% carbon and is made by burning hydrocarbons like oil, wax, kerosene, paraffin, or petrol in a limited amount of air.
- The smoke from the burning is cooled on a surface, and the fine black powder (soot) is collected.
- In India, this black powder is called kajal and is used in many ways.
Uses of Lampblack
- It is used as a pigment (colouring agent) in printer’s ink, carbon paper, black paint, and shoe polish.
- It is used to harden rubber tyres.
- It is used to make gunpowder.
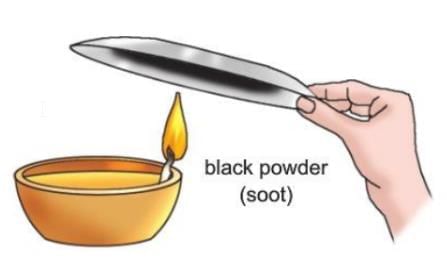
Activity 9.7
Aim: To prepare kajal from vegetable oil.
Materials required: Some mustard oil or ghee, an earthen lamp (diya), a cotton wick, a metal plate.
Procedure:
- Take some mustard oil or ghee in an earthen lamp (diya) and put a cotton wick in it.
- Light the cotton wick.
- Hold a metal plate over the flame as shown in the figure.
Observation: You will see that a black powder (soot) collects on the surface of the plate.
Conclusion: The black powder formed is called kajal.
Coal
- Coal is a black or dark-brown rock that burns and is found deep under the ground in mines.
- It is made of hydrocarbons, some free carbon, and small amounts of nitrogen and sulphur.
Formation of Coal
- Coal was formed millions of years ago from ancient plants that got buried under the ground.
- These plants were covered by soil and rocks, and bacteria broke them down over time.
- New plants grew on top, and events like volcanoes and earthquakes buried them deeper.
- Over many years, high temperature and pressure turned the dead plants into coal.
- This slow process of turning plants into coal is called carbonisation.
Fun Fact
Carbonisation is a very slow process that can take thousands of years to turn dead plants into coal.
Types of Coal
There are four types of coal based on how much carbon they have:
- Peat:
- It is the first stage of coal formation.
- It is made from partly decayed leaves, twigs, and branches in swamps with a lot of moisture.
- It has a low carbon content, about 50–60%, and is light brown in colour.
- It is the youngest type of coal, burns slowly, gives off a bad smell, and leaves a lot of ash and residue.
- Lignite:
- It is the next stage after peat and is also called brown coal.
- It is made of compressed woody matter that has lost most of its moisture.
- It has more carbon than peat, about 60–70%, but still crumbles easily.
- It burns easily but gives off a lot of ash and residue.
- Bituminous:
- It is formed when lignite gets more compressed and turns into a dense, dark, and brittle coal.
- It has a higher carbon content, about 75–80%.
- It is the most common type of coal but produces ash and sulphur compounds that pollute the air when burnt.
- Anthracite:
- It is the final stage of coal formation and is also called hard coal.
- It has the highest carbon content, over 90–95%, and burns with very little smoke.
- It burns for a long time but gives less heat compared to bituminous coal.
- The more carbon a coal has, the more heat it gives; so, anthracite gives the most heat, and peat gives the least.
Uses of Coal
- Coal is used as a fuel in homes and industries to produce heat and energy.
- It is used to make synthetic petroleum products like coal gas, coal tar, and ammonia solution.
- It is a source of chemicals like benzene, naphthalene, aniline, and phenol, which are used in many products.
- Coal is used to make fertilisers, drugs, synthetic textiles, and perfumes.
Destructive Distillation of Coal
When coal is heated strongly without air, it breaks down into different products; this process is called destructive distillation.
The equation for this process is:

- Coke: It is a tough, porous, and black solid with about 98% carbon. It is used to make steel and to get metals like zinc and iron from their ores.
- Coal gas: It is a mix of hydrogen, carbon monoxide, and methane. It burns with a bluish flame and is used as a fuel in homes and industries.
- Coal tar: It is a thick, black liquid that smells bad. It is used to make dyes, drugs, explosives, and other things.
- Ammonia solution: It is ammonium hydroxide and is used to make fertilisers like ammonium sulphate and ammonium superphosphate.
Carbon Dioxide
- Carbon dioxide (CO₂) is a colourless gas that makes up about 0.04% of the air by volume.
- It is a greenhouse gas that traps heat and is formed during burning of fuels and breathing by living things.
- Plants use carbon dioxide to make food through a process called photosynthesis, discovered by Jan Baptista van Helmont.
- Joseph Black and Antoine Lavoisier also studied the role of carbon dioxide in breathing.
Preparation of Carbon Dioxide
Carbon dioxide can be made in these ways:
- By reacting dilute acids with metal carbonates.
- By burning carbon.
- By heating metal carbonates or metal hydrogen carbonates.
By the Reaction of Dilute Acids with Metal Carbonates
Dilute acids like dilute hydrochloric acid (HCl) react with calcium carbonate to form carbon dioxide.
The equation is:
Method:
- Take a few chips of marble (calcium carbonate, CaCO₃) in a Woulfe’s bottle, which has two necks.
- Add a few drops of dilute hydrochloric acid through a thistle funnel.
- You will see fizzing when the acid reacts with marble and carbon dioxide is released.
- Carbon dioxide is heavier than air, so it is collected by pushing air upward in a glass jar (upward displacement).
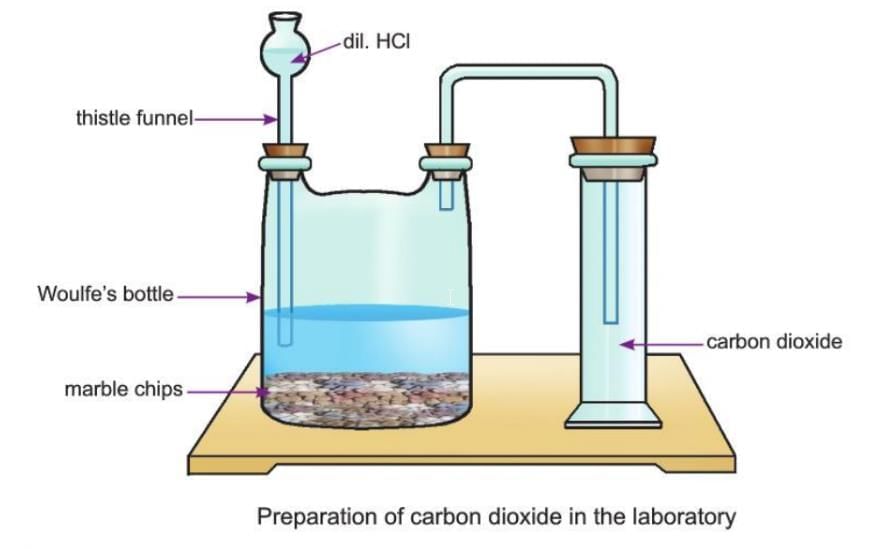
By Burning Carbon
When fuels with carbon are burnt, they release carbon dioxide.
The equations are: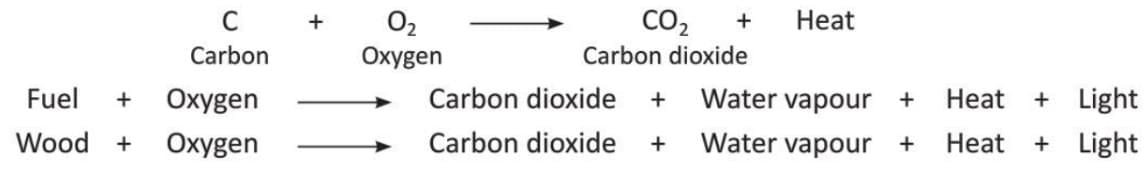
By Heating Metal Carbonates or Metal Hydrogen Carbonates
Metal carbonate and metal hydrogen carbonate produce carbon dioxide when heated.
Tests for Carbon Dioxide
We can do tests to check if carbon dioxide is present:
- When carbon dioxide is passed through lime water, the lime water turns milky because calcium carbonate forms, which does not dissolve in water. If more carbon dioxide is passed, the milkiness goes away because calcium bicarbonate forms, which dissolves in water.
- When a burning candle or splinter is put in a jar with carbon dioxide, the flame goes out because carbon dioxide does not help things burn.
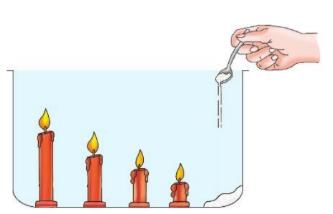
Activity 9.8
Aim: To show that carbon dioxide is heavier than air and does not support burning.
Materials needed: A big glass container, 4-5 candles of different heights, baking soda, vinegar.
Procedure:
- Fix the candles of different sizes in the container.
- Add a heaped tablespoon of baking soda in the container.
- Light the candles.
- Pour vinegar, one tablespoon at a time, on the baking soda.
- Keep adding vinegar to the baking soda until the last candle goes out.
- Light another candle outside the container and pour the invisible gas from the container on the lighted candle.
Observation: The candle flames in the container go out one by one, starting from the shortest to the tallest. The candle outside the container also goes out when the invisible gas is poured on it.
Conclusion: Carbon dioxide, made by the reaction of vinegar and baking soda, puts out the flames because it does not support burning. The shortest candle goes out first because carbon dioxide is heavier than air and settles in the lower part of the container. Carbon dioxide can be poured out like water because it is heavier than air.
Properties of Carbon Dioxide
Physical Properties
- Carbon dioxide is a gas with no smell and no color.
- It is slightly acidic and can dissolve a little in water.
- It is not poisonous, but if there is too much carbon dioxide in the air, it can cause suffocation (difficulty in breathing).
- When cooled to -78°C, carbon dioxide turns into a snow-white solid called dry ice.
- Dry ice changes directly from a solid to a gas without becoming a liquid; this is called sublimation.
- Dry ice is used in making products like plastics, chemicals, medicines, drinks, and metals.
- Carbon dioxide is heavier than air.
Chemical Properties
- Carbon dioxide is made by the strong bonding of carbon and oxygen.
- It does not help things burn.
- It turns moist blue litmus paper red, which shows that carbon dioxide is acidic.
- Carbon dioxide mixes with water to form carbonic acid.
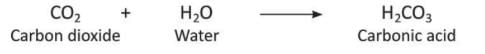
- Carbon dioxide reacts with alkalis (bases) to form salt and water.
- When carbon dioxide reacts with sodium hydroxide (NaOH), it forms sodium carbonate (Na₂CO₃) and water (H₂O).

- When carbon dioxide reacts with potassium hydroxide (KOH), it forms potassium carbonate (K₂CO₃) and water (H₂O).

- When too much carbon dioxide is passed through alkalis, it forms a soluble bicarbonate.
- When too much carbon dioxide reacts with sodium carbonate (Na₂CO₃) and water (H₂O), it forms sodium bicarbonate (2NaHCO₃).
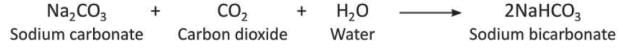
- When too much carbon dioxide reacts with potassium carbonate (K₂CO₃) and water (H₂O), it forms potassium bicarbonate (2KHCO₃).
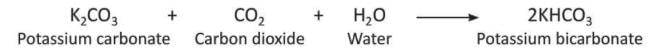
- When carbon dioxide is passed through lime water (Ca(OH)₂), it turns milky because calcium carbonate (CaCO₃) forms, which does not dissolve in water.
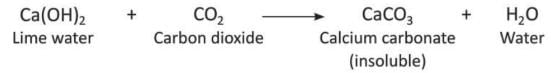
- If more carbon dioxide is passed, the milkiness goes away because calcium bicarbonate (Ca(HCO₃)₂) forms, which dissolves in water.

- Carbon dioxide reacts with metal oxides to form metal carbonates.
- When carbon dioxide reacts with sodium oxide (Na₂O), it forms sodium carbonate (Na₂CO₃).
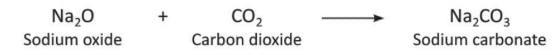
- When carbon dioxide reacts with magnesium oxide (MgO), it forms magnesium carbonate (MgCO₃).
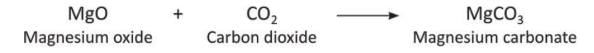
Uses of Carbon Dioxide
- Aerated drinks:
- Carbon dioxide is used to make aerated drinks like soda water.
- When dissolved in water, it forms carbonic acid, which gives a sharp, sour taste.
- Sugar, colour, and flavour are added to soda water to make soft drinks.
- Dry ice:
- Solid carbon dioxide is called dry ice.
- It acts as a good refrigerant (keeps things cold) and is used to preserve food items like fruits, vegetables, meat, and grains.
- It prevents insect attacks and keeps food fresh for a long time.
- Fertilisers:
- Carbon dioxide and ammonia are heated at 200°C under high pressure to make urea, an important fertiliser.
- Carbogen:
- Carbogen is a mix of 5% carbon dioxide and 95% oxygen.
- It is used for artificial respiration and given to patients with pneumonia, poisoning, or drowning.
- Washing soda and baking soda:
- Carbon dioxide is used to make washing soda (sodium carbonate) and baking soda (sodium bicarbonate).
- Baking industry:
- Carbon dioxide is used in the baking industry to make dough rise and become light.
- Baking powder is a mix of baking soda and potassium hydrogen tartrate.
- When baking powder is added to dough, the baking soda reacts to produce carbon dioxide, which makes the dough rise.
- When the gas rises through the dough, it creates spaces, making cakes and bread light, soft, and spongy.
- Fire extinguishers:
- Carbon dioxide is used in fire extinguishers because it does not burn and stops oxygen from reaching the fire.
- Photosynthesis:
- Green plants use carbon dioxide to make food through photosynthesis.
Activity 9.9
Aim: To observe the use of carbon dioxide in cooking.
Materials required: A bowl, some wheat flour, some baking soda, some water, some vinegar.
Procedure:
- Take some wheat flour and add a tablespoon of baking soda to the flour.
- Add water and knead properly to make a dough so that the baking soda is evenly mixed.
- Mark the height of the dough.
- Add a little vinegar to the dough and mix it properly.
- Leave the dough for 7 to 9 minutes.
Observation: You will observe that the dough rises.
Conclusion: Baking soda has carbonate, and vinegar has acetic acid. They react to produce carbon dioxide, which escapes through the pores in the dough, making the dough rise.
Greenhouse Effect
- The greenhouse effect is when gases in the earth’s atmosphere trap heat from the sun and keep the earth warm.
- Carbon dioxide is a greenhouse gas that absorbs heat from the sun’s rays.
- The sun’s rays reach the earth and warm it, but some heat is sent back to space.
- Carbon dioxide in the air traps some of this heat, keeping the earth warm so life can survive.
- Other gases like methane and water vapour also help in the greenhouse effect.
- Human activities like burning fossil fuels and using many vehicles release a lot of carbon dioxide, which increases the greenhouse effect and causes global warming.
Global Warming
- Global warming is the increase in the earth’s average temperature due to too much greenhouse gases.
- It changes weather patterns and causes climate change, leading to more rain, higher sea levels, droughts, and floods.
- It also causes the melting of polar ice caps.
Effects of Global Warming
- Melting glaciers: The rise in temperature causes glaciers to melt, leading to floods in the future.
- Climate change: Global warming changes weather patterns, making it hard for plants and animals to survive. It may cause plants to die, leading to a shortage of food for animals and humans.
- Droughts: Warmer temperatures cause large-scale evaporation of water, leading to droughts in some areas.
- Agriculture: Global warming affects crop growth, making it harder to grow food, which can lead to food shortages.
Causes of Increased Concentration of Carbon Dioxide in the Atmosphere
- Cutting down of trees (deforestation): Trees absorb carbon dioxide, so cutting them reduces the amount of CO₂ removed from the air.
- Air pollution: Burning fuels, industrial activities, and using chemical weapons release more carbon dioxide into the air.
Measures to Balance the Concentration of Carbon Dioxide in the Atmosphere
- Plant more trees and use renewable energy sources like solar and wind power.
- Use less fossil fuels to reduce carbon dioxide emissions.
- Use filters in the chimneys of factories and powerhouses to trap harmful gases.
Carbon Monoxide
- Carbon monoxide is made of carbon and oxygen, and its formula is CO.
- It is a very poisonous and flammable gas.
- It is found in small amounts in coal gas in coal mines.
- It is made during volcanic eruptions and when charcoal burns with less oxygen.

- It is also produced in ovens at home and industries, and from car engines that use petrol or diesel.
Properties of Carbon Monoxide
- It is a gas with no color and no smell, so it is hard to notice.
- It does not mix well with water.
- It burns in air with a blue flame and makes carbon dioxide.
- It is a reducing agent, which means it takes oxygen away from metal oxides to make pure metals.
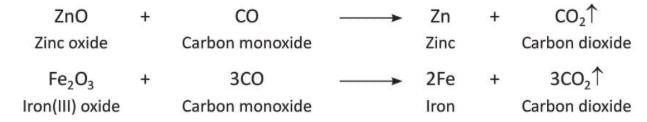
- This property helps in taking out metals like zinc and iron from their ores.
Poisonous Nature of Carbon Monoxide
- Carbon monoxide is a very poisonous gas.
- Even 1% of carbon monoxide in the air can kill a person in a few minutes.
- When we breathe it, it mixes with haemoglobin in our blood to form carboxyhaemoglobin.
- Carboxyhaemoglobin stops oxygen from reaching different parts of the body.
- This causes a problem called asphyxia, which can lead to suffocation, unconsciousness, or death.
- It is dangerous to sleep in a closed room with burning coal because carbon monoxide can form.
- Running a car engine in a closed garage is also risky for the same reason.
Precautions to Be Taken to Avoid Carbon Monoxide Poisoning
- Do not sleep in a closed room where coal is burning.
- Do not run a car engine in a closed space like a garage.
- Do not sleep near lime kilns because the air around them may have carbon monoxide.
- Stay away from burning tobacco because even small amounts of carbon monoxide are harmful.
- Carbon monoxide is dangerous for the breathing system of smokers and people who breathe in the smoke.
- In places with high carbon monoxide, wear gas masks that can absorb it and turn it into carbon dioxide.
In Case of Carbon Monoxide Poisoning:
- Take the person to an open area right away.
- Give them artificial respiration with a mix of 95% oxygen and 5% carbon dioxide.
How Is Carbon Monoxide Added to the Atmosphere?
- Carbon monoxideis added by incomplete burning of fuels.
- When fuels like coal, coke, or charcoal burn with less air or oxygen, carbon monoxide is made.
- Air or oxygen reacts with carbon to form carbon dioxide, but with less oxygen, it forms carbon monoxide.
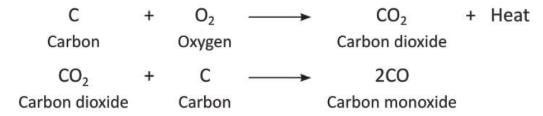
- Exhaust fumes from fuels with hydrocarbons also make carbon monoxide.
- This happens in car engines when there is not enough air, like in automobiles.
- In India, 50-55% of fuel is wood, which burns incompletely and makes harmful smoke.
- The government is working to provide cleaner fuel like LPG through schemes like Pradhan Mantri Ujjwala Yojana (PMUY).
- PMUY helps poor families get clean cooking fuel.
Points To Remember
- Many things we use every day are made of carbon and its compounds.
- Carbon exists in both free and combined forms and is known to have more than 5 million compounds.
- Carbon compounds are divided into two types: carbon compounds of biological origin and carbon compounds of non-biological origin.
- A big reason for so many carbon compounds is their valency.
- Carbon has two allotropic forms: crystalline and amorphous.
- Crystalline forms of carbon are diamond, graphite, and fullerene.
- Amorphous forms of carbon are charcoal, coke, gas carbon, lamp black (carbon black or soot), and coal.
- Diamond is the purest form of carbon and the hardest natural substance known.
- Graphite is a greyish-black opaque substance.
- Buckminsterfullerene is a spherical, crystalline, allotropic form of carbon with 60 carbon atoms.
- Charcoal is made by the destructive distillation of things like wood, bones, and sugar.
- There are three types of charcoal: wood charcoal, bone or animal charcoal, and sugar charcoal.
- Coke is a greyish-black solid that burns without smoke.
- Gas carbon is a greyish substance made by the destructive distillation of coal, also called petroleum carbon.
- Lampblack is a fine powder made by burning hydrocarbons like oil, wax, kerosene oil, paraffin, naphthalene, or petrol in limited air.
- Coal has different types: peat, lignite, bituminous, and anthracite.
- Carbon monoxide is a very poisonous gas and is made when carbon burns with less oxygen.
Glossary
- Organic compounds: Compounds of carbon that come from living things.
- Allotropy: When an element can exist in different physical forms but has the same chemical properties.
- Destructive distillation: The process in which break down of an organic substance takes place when it is heated strongly in the absence of air.
- Wood charcoal: A type of charcoal which is black, porous and brittle and is obtained by destructive distillation of wood.
- Wood gas: The combustible mixture of carbon dioxide, carbon monoxide, methane and hydrogen.
- Activated charcoal: A type of charcoal formed by heating wood charcoal at around 900°C.
- Bone charcoal: A type of granular black substance that contains only 10% carbon and a high percentage of impurities like calcium phosphate and calcium carbonate.
- Sugar charcoal: The purest available amorphous form of carbon prepared by destructive distillation of cane sugar or glucose.
- Coal: A complex mixture of hydrocarbons and some free carbon.
- Dry ice: Solid carbon dioxide.
- Carboxyhaemoglobin: A mixture of 5% carbon and 95% oxygen monoxide on entering the human blood system combines with haemoglobin present in the blood.
- Carbonisation: The slow chemical process of conversion of dead and decaying matter into coal.
|
9 videos|37 docs|9 tests
|
FAQs on Carbon and Its Compounds Chapter Notes - Chemistry Class 8 ICSE
| 1. What are the different allotropes of carbon? |  |
| 2. What are the chemical properties of carbon compounds? |  |
| 3. How does carbon dioxide affect the environment? |  |
| 4. What is the difference between carbon monoxide and carbon dioxide? |  |
| 5. What are the uses of amorphous carbon? |  |