Elements, Compounds and Mixtures Chapter Notes | Chemistry for EmSAT Achieve PDF Download
Introduction
In this chapter, we will learn about the tiny building blocks of everything around us, called matter. Matter can be in different forms like solid, liquid, or gas because of how its tiny parts are arranged. We will study what elements and compounds are, how they are different from mixtures, and how we can separate mixtures. We will also learn about the symbols of elements and how to use them.
Pure Substances
- Substances that have only one type of particle are called pure substances.
- Pure substances are of two types:
- Elements are made of only one type of atom.
- Compounds are made of only one type of molecule.
- Both elements and compounds have the same properties like melting point, boiling point, and specific gravity.
- Elements and compounds are both homogeneous, which means they have a uniform composition.
Elements
- Matter is made of basic substances called elements.
- An element cannot be broken into simpler substances by any physical or chemical method.
- Some examples of elements are gold, silver, mercury, sulphur, hydrogen, and oxygen.
- There are 118 known elements today.
- Out of these 118 elements, 92 are found naturally, and 26 are made artificially.
- An element is made of tiny particles called atoms, which show all the properties of that element.
- An atom is the smallest part of an element that has all its properties.
- Some atoms can exist on their own, while others are found combined with atoms of the same or different elements.
- For example, a sodium atom can exist alone, but a hydrogen atom combines with another hydrogen atom to form a molecule.
- A molecule is the smallest unit of a pure substance that can exist on its own without losing its basic nature or identity.
Characteristics of an Element
- An element cannot be broken into simpler substances by physical or chemical methods because it is the basic form of matter.
- An element is a pure substance as it has atoms of only one kind.
- An element can exist on its own or in combination with other elements to form compounds.
- Elements exist in all three states of matter: solid, liquid, and gas.
Classification of Elements
Elements can be divided into metals, non-metals, metalloids, and noble gases based on their properties.
Metals
- Most elements we know are metals.
- Examples of metals are gold, silver, iron, copper, platinum, aluminium, tin, zinc, sodium, potassium, lead, and calcium.
- Most metals are found in the Earth's crust.
- Some metals are also found in seas and oceans.
Properties of Metals
- Metals are usually hard solids.
- Metals have a shiny, lustrous appearance.
- Metals are good conductors of heat and electricity.
- Metals have high melting and boiling points.
- Metals are malleable and ductile.
- Metals are sonorous, which means they produce a sound when struck.
Non-Metals
- Non-metals are found in abundance in nature.
- Non-metals are mostly found in the Earth's crust, oceans, and atmosphere.
- Examples of non-metals are hydrogen, oxygen, nitrogen, chlorine, phosphorus, and sulphur.
Properties of Non-Metals
- Non-metals can be either soft solids or gases.
- Non-metals are mostly brittle in nature, which means they break easily.
- Non-metals do not have a shiny or lustrous appearance.
- Non-metals are bad conductors of heat and electricity.
- Non-metals are neither malleable nor ductile.
- Non-metals have low melting and boiling points.
- Non-metals are not sonorous, which means they do not produce a sound when struck.
Common Elements
- In the universe, the common elements are hydrogen and helium.
- In the Earth's crust, the common elements are oxygen, followed by silicon, aluminium, and iron.
- In the atmosphere, the common element is nitrogen.
- In the human body, the common elements are carbon, hydrogen, and oxygen, which make up the compounds in cells.
Metalloids
- Some elements have properties of both metals and non-metals, and they are called metalloids.
- Examples of metalloids are boron, silicon, arsenic, and antimony.
- Metalloids are solid, shiny, or dull.
- Metalloids are ductile and malleable.
- Metalloids conduct heat and electricity better than non-metals but not as well as metals.
Noble (Inert) Gases
- Some elements are present in a gaseous state and do not react chemically with other elements.
- These elements are called noble or inert gases.
- Examples of noble gases are helium, neon, argon, krypton, xenon, and radon.
- Noble gases are present only in traces in the air.
Fun Fact
- Aluminium is the most common metal found in the Earth's crust.
- Iron makes up a large part of the Earth's core.
- Metals have a property called malleability, which means they can be beaten into thin sheets.
- Metals have a property called ductility, which means they can be drawn into wires.
- Bromine is the only non-metal that is liquid at room temperature.
- Mercury is the only metal that is liquid at room temperature.
- In the universe, hydrogen and helium together make up about 99% of the mass.
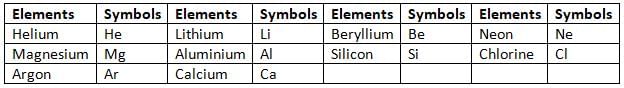
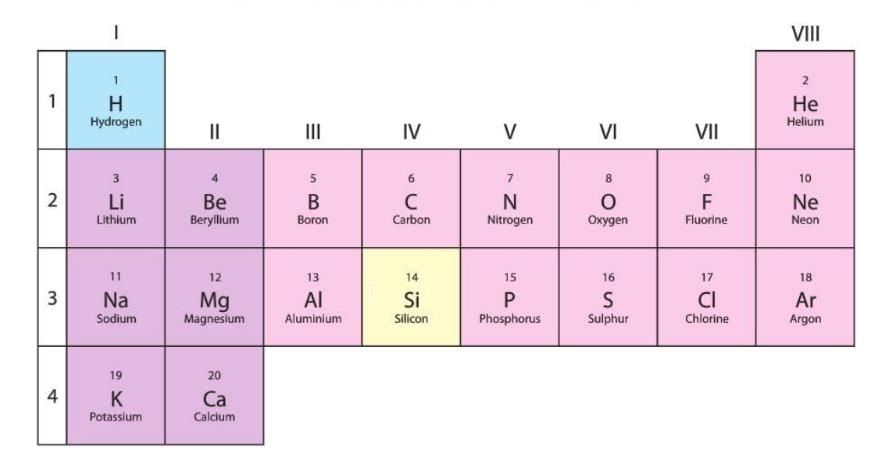
First 20 Elements in Periodic Table
Symbols of Elements
- Writing the full names of elements while describing their reactions is not easy.
- So, scientists decided to use short forms called symbols to represent elements.
- A symbol of an element is one or two letters chosen by the International Union of Pure and Applied Chemistry (IUPAC).
- A symbol represents one atom of an element.
Writing Symbols of an Element
- Some elements are represented by the first letter of their names.
- For example, the symbol for carbon is C, for nitrogen is N, for oxygen is O, and for hydrogen is H.
- When there are more than one element starting with the same letter, only one element is represented by the first letter of its name.
- The other elements are represented by the first two letters of their names.
- The first letter is always in uppercase, and the second letter is always in lowercase.
- For example, boron is represented by B, while barium, beryllium, bismuth, and bromine are represented by Ba, Be, Bi, and Br.
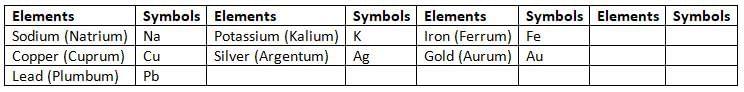
- For some elements, the symbol comes from their Latin names.
- For example, the Latin name of copper is cuprum, and its symbol is Cu.
- The Latin name of potassium is kalium, and its symbol is K.
Symbols of Some Common Elements are Given in Tables
Symbols of Elements Derived from First Letter of their Names
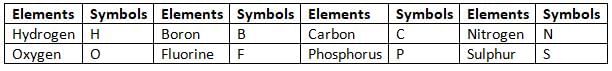
Symbols of Elements Derived from Two Letters of their Names
Symbols of Elements Derived from their Latin Names
Compounds
- Many elements combine with each other to form compounds.
- A compound is a pure substance made by the chemical combination of two or more elements in a definite proportion by mass.
- For example, common salt is a compound made of two elements: sodium and chlorine.
- A compound can be broken down into its elements.
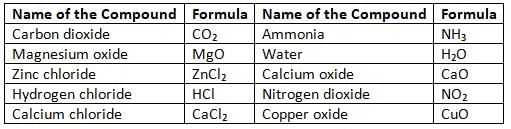
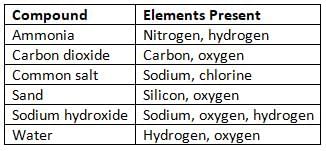
Some Common Compounds
Characteristics of a Compound
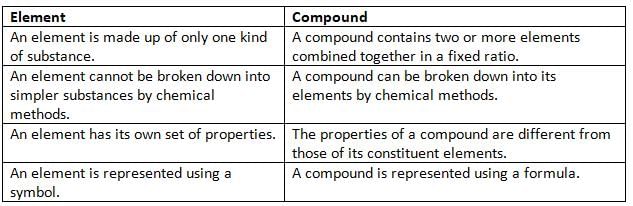
- A compound is made up of two or more elements.
- A compound always contains the same elements combined together in a fixed ratio.
- For example, the ratio of water is always 2 : 1, which means it has two hydrogen atoms and one oxygen atom.
- A compound is a pure and homogeneous substance.
- The properties of a compound are different from the properties of its individual elements.
- For example, hydrogen and oxygen are gases, but when they combine to form water, it becomes a liquid.
- The constituents of a compound cannot be separated by physical methods like filtration.
- For example, we cannot separate oxygen and hydrogen from water by simple means like boiling.
- A compound can be broken down into its elements by chemical methods.
- For example, when electricity is passed through water mixed with an acid, it breaks down into hydrogen and oxygen.
- Energy is either absorbed or given out when a compound is formed.
- For example, energy is produced in the form of heat and light when hydrogen and oxygen combine to form water.
Differences between Elements and Compounds
Atoms and Molecules
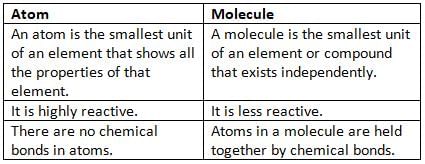
Atom
- An atom is the tiniest part of an element that has all the features of that element.
- Some atoms can exist alone, like atoms of noble gases, which are very stable.
- Other atoms, like hydrogen or sodium atoms, cannot stay alone and join with other atoms to form molecules.
Characteristics of an Atom
- Atoms are very active and usually join with other atoms of the same element or different elements to form molecules.
- For example, hydrogen atoms join with other hydrogen atoms or atoms of other elements.
- Noble gases, like helium, have atoms that can exist alone because they are very stable.
- All atoms of the same element are the same and have the same features.
- Atoms of different elements have different features.
- The features of an atom stay the same during physical and chemical changes.
- For example, if you break a piece of iron into smaller pieces, those pieces will still be attracted to a magnet because they are still iron atoms.
Did You Know?
John Dalton was a scientist from England. He created the modern atomic theory. His theory said that all matter is made of tiny particles called atoms, which cannot be divided further.
Molecule
A molecule is the smallest part of an element or a compound that can exist alone and still have all the features of that substance.
Characteristics of a Molecule
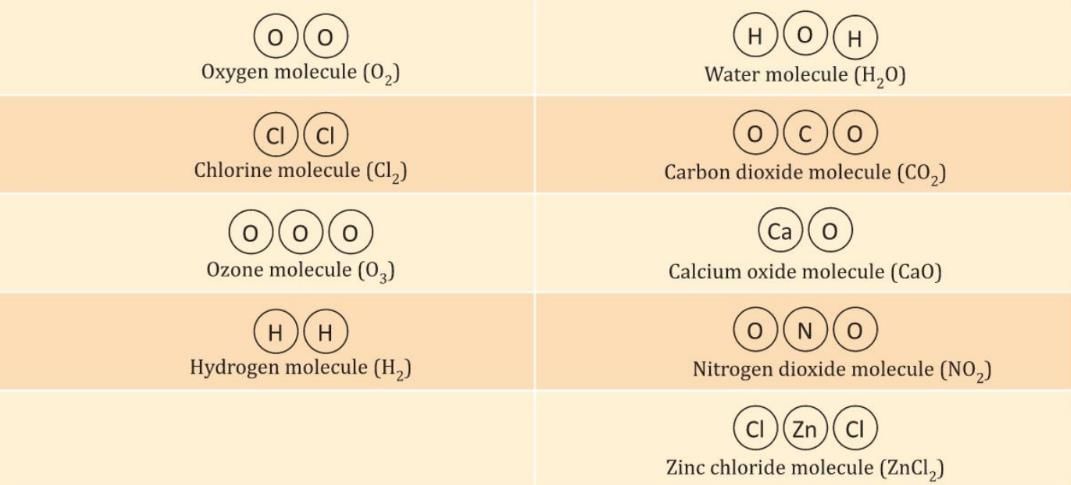
- Atoms of the same element or different elements join together to form molecules.
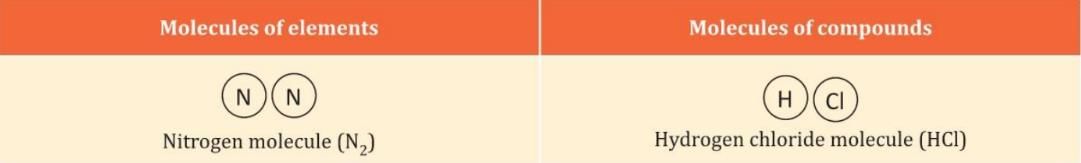
- For example, when two hydrogen atoms join, they form a hydrogen molecule (H₂).
- When atoms of two or more different elements join, they form a molecule of a compound.
- In a molecule, atoms of different elements are always in a fixed whole number ratio.
- For example, in a water molecule (H₂O), there are always 2 hydrogen atoms for 1 oxygen atom.
- Atoms in a molecule are held together by chemical bonds.
- A chemical bond is the force that holds atoms together to make a molecule.
Diagrammatic Representation of Some Molecules
Molecular Formula
A molecular formula shows the total number and type of atoms in a molecule of a compound.
Molecular Formulae of Some Compounds
Differences between an Atom and a Molecule
Uses of Some Elements and Compounds
- Copper is a good conductor of heat and electricity and is used to make electrical wires.
- Tungsten has a high melting point and is used to make filaments of light bulbs.
- Silver, platinum, and gold are shiny and attractive metals used to make jewelry, iron, and steel, which are widely used in the construction of buildings and are also used to make tools and machinery.
- Coal, wood, and natural gas are used as fuels because they produce heat energy on burning.
- Utensils used for cooking are made from metals like steel, copper, and aluminum, as they are good conductors of heat.
- Metals like iron and copper are sonorous and are used to make bells.
- Plastic is a bad conductor of heat and electricity and is used to insulate wires and is also used to make non-stick cookware.
- Carbon dioxide is used in fire extinguishers because it does not support combustion.
- Sand is used in the preparation of glass.
- Chlorine is used as a disinfectant.
- Argon is used in light bulbs because it does not react with metals at high temperatures.
- Graphite is used to make lead in pencils and is also used to prepare lubricants.
- Diamond is a form of carbon and is the hardest naturally occurring substance; it is used to cut glass and is considered the most precious gem; impure diamond is used to cut glass.
Mixtures
- A mixture is a combination of two or more elements or compounds in any proportion.
- In a mixture, there is no chemical change, so the original atoms and molecules remain as they are.
- The parts of a mixture are loosely held together, so they keep their own properties and can be present in any proportion.
- For example, lemonade is a mixture of water, salt, sugar, and lemon juice; when we drink lemonade, we can taste all its parts.
- Mixtures have different kinds of molecules.
- For example, milk is a mixture of fats, carbohydrates, proteins, salts, vitamins, and water.
Characteristics of a Mixture
- The parts of a mixture can be present in any ratio.
- The composition of a mixture can be changed by changing the amount of its parts.
- For example, in a mixture of sugar and water, you can have one spoonful of sugar and two spoonfuls of water, or three spoonfuls of sugar and one spoonful of water; both are mixtures, but the amount of sugar changes.
- The parts of a mixture keep their original properties.
- For example, the taste of sugar is sweet, and it stays the same even when mixed with water; the mixture has no new properties of its own.
- There is no fixed melting or boiling point for a mixture.
- For example, the boiling point of a mixture of one glass of water and one spoonful of sugar will be different from the boiling point of a mixture of the same amount of water and four spoonfuls of sugar.
- A mixture can be separated into its parts by simple physical or mechanical means.
- For example, we can separate salt from iron dust using a magnet.
- Generally, no energy is absorbed or released when a mixture is formed.
Types of Mixtures
We come across different types of mixtures in our daily lives. In some mixtures, the components can be easily distinguished, while in others, the components are not clearly visible.
Mixtures are divided into two main types:
1. Homogeneous Mixture
- A homogeneous mixture is a mixture where the components cannot be seen separately and are uniformly distributed throughout its volume.
- For example, salt solution and sugar solution are homogeneous mixtures.
2. Heterogeneous Mixture
- A heterogeneous mixture is a mixture where the components can be easily seen and are not uniformly distributed.
- For example, mixtures of sand and gravel or oil and water are heterogeneous mixtures.
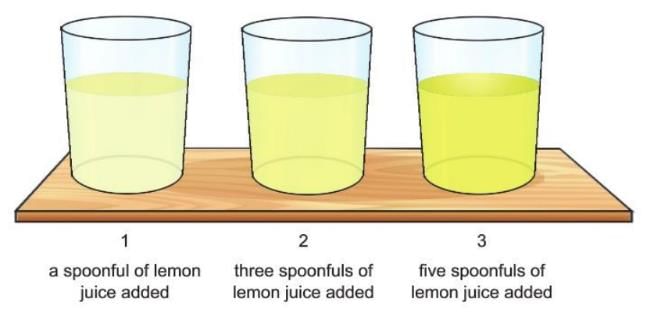
Activity 2.4
Aim: To show that the properties of a mixture depend on the quantity of the components present.
Materials required: Some drinking water, 3 glass tumblers, one spoon, 9 spoonfuls of lemon juice.
Procedure:
- Take three glass tumblers and fill half of each with drinking water.
- Add a spoonful of lemon juice in the first tumbler and stir to mix.
- Add three spoonfuls of lemon juice in the second tumbler and stir to mix.
- Add five spoonfuls of lemon juice in the third tumbler and stir to mix.
- Taste the three solutions one by one.
Observation: All the samples are sour; however, the degree of sourness varies.
Conclusion: In all three cases, although a homogeneous solution is obtained, the property differs depending upon the concentration of the constituents present.
Differences between Compounds and Mixtures
- Let’s understand the differences between compounds and mixtures with the help of iron powder and sulphur powder.
- First, when iron powder and sulphur powder are mixed and then heated, a material iron sulphide (FeS) is formed.
- Iron sulphide is an entirely different product with different properties from iron and sulphur.
- Iron and sulphur combine chemically on heating in the ratio of 7 : 4 by mass to form iron sulphide.
- Thus, in iron sulphide (FeS),

- Again, when iron powder and sulphur powder are mixed and no heat is given, they do not react to form any other substance.
- Thus, they form a mixture of two substances that can be separated easily.
- Iron powder can be separated by using a bar magnet because iron gets attracted by a magnet, while the sulphur powder left behind does not get attracted; sulphur is soluble in carbon disulphide, whereas iron sulphide is neither attracted by a magnet nor soluble in carbon disulphide.
Differences between Compounds and Mixtures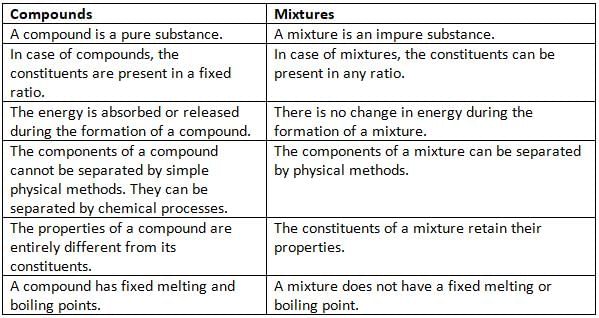
Why is Water a Compound and Air a Mixture?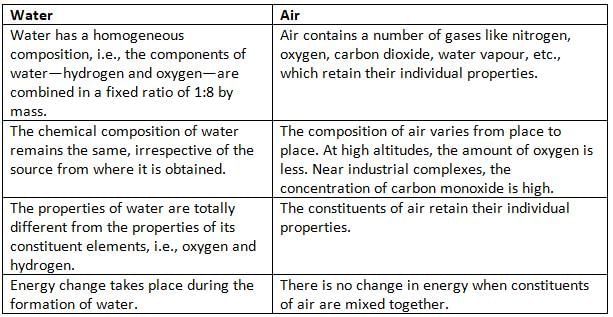
Separation of Mixtures
The act of isolating the elements of a mixture is known as the separation of mixtures. We perform this process in our everyday lives to extract the components we need.
Need for Separation of Mixtures
Isolating different components of a mixture can be crucial because the mixture might include unwanted or harmful substances. The points below explain why separation of mixtures is necessary:
- To eliminate harmful or unwanted substances: Food products like grains, legumes, and spices might contain impurities such as stones, husks, insects, and their eggs, which can be harmful if consumed. Thus, these need to be removed before cooking to get rid of the undesirable substances present.
- To obtain a useful substance: Separating mixtures is essential when we want to extract pure substances from them. For instance, when crude petroleum oil is separated, we obtain useful products like petrol, diesel, kerosene oil, and wax.
- To acquire a pure form of a substance: Many substances need to be in their pure form for purposes like making medicines, conducting experiments in labs, etc. For example, impurities must be removed from water because pure water is required for preparing medicines, in laboratories, and for car batteries.
The Principle of Separation
- The way we separate a mixture depends on what type of mixture it is and the properties of its parts.
- Some mixtures, like sugar in water, can be seen as a solution because the parts mix completely.
- In such mixtures, we cannot see the parts separately.
- But in other mixtures, like sand in water, the parts do not mix fully, and we can see them.
- The separation method also depends on the size of the particles in the mixture.
- For example, we can separate iron filings and sulphur using a magnet because iron gets attracted to the magnet, but sulphur does not.
Sieving
- Sieving is a method to separate parts of a mixture that are different in size.
- We use a sieve, which is a mesh placed over a frame, like the one your mother uses to sift flour.
- During sieving, small particles pass through the holes of the sieve, but bigger particles stay on top.
- For example, at construction sites, sieves are used to separate stones and pebbles from sand.
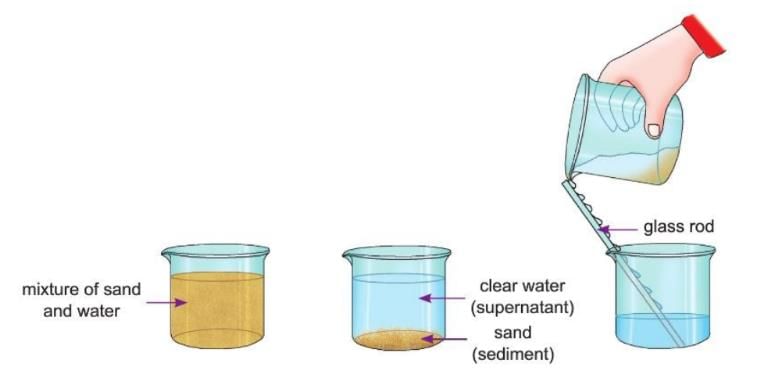
Sedimentation and Decantation
- When a mixture of an insoluble substance and water is left undisturbed for some time, the insoluble particles settle down at the bottom.
- This process is called sedimentation, and the settled particles are called sediment.
- The clear liquid above the sediment is called supernatant liquid.
- We can then pour the clear liquid into another beaker without disturbing the sediment.
- This pouring process is called decantation.
- Sedimentation and decantation are used to separate a solid and a liquid where the solid is heavier than the liquid and does not dissolve in it.
- For example, we can separate a mixture of sand and water using this method.
Activity 2.5
Aim: To separate a mixture of sand and water by sedimentation and decantation.
Materials required: 2 beakers, some sand, some water, a glass rod.
Procedure 1:
- Take a mixture of sand and water in a beaker.
- Stir it and keep it undisturbed for 15-20 minutes.
- You will observe that sand settles down at the bottom of the beaker, leaving clear water over it.
- This process is called sedimentation.
Procedure 2:
- Now take another beaker and put a glass rod in it.
- Pour the clear water gently from the first beaker into the second beaker along the glass rod, as shown in the figure.
Observation: You will observe that the sand and water get separated. This process is called decantation.
Limitations of Sedimentation and Decantation
- The parts of an insoluble solid and liquid cannot be completely separated using this method.
- If the solid is lighter than the liquid, it will not settle down, so this method cannot be used.
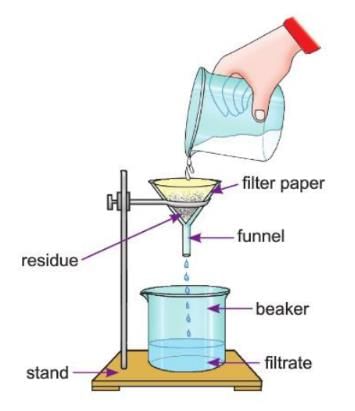
Filtration
- Filtration is a method to separate an insoluble solid from a liquid.
- In this method, the mixture is passed through a filter paper.
- The filter paper has tiny pores that allow only the liquid to pass through.
- The insoluble solid stays behind on the filter paper and is called the residue.
- The clear liquid that passes through the filter paper is called the filtrate.
- We can also use materials like glass wool, charcoal, sand, unglazed porcelain, cotton, or a strainer instead of filter paper.
- For example, we use filtration at home to separate tea leaves from tea using a strainer.
- Filtration is also used to remove pulp from fresh juice.
Did You Know?
Filter paper is a special kind of paper with tiny pores of a specific size. It allows only liquid to pass through, while undissolved solid particles are retained on the filter paper.
Activity 2.6
Aim: To separate chalk powder and water by filtration.
Materials required: Chalk powder, some water, 2 beakers, filter paper, a funnel, a stand.
Procedure:
- Take a mixture of water and chalk powder in a beaker.
- Take a circular filter paper and fold it to make a cone, as shown in the figure.
- Place this cone in a funnel and fix the funnel on the clamp of an iron stand.
- Place another beaker below the funnel.
- Pour the mixture of chalk and water slowly into the funnel.
- Make sure the mixture does not overflow from the funnel.
Observation: You will observe that clear water (filtrate) drips into the beaker, and the chalk powder is left behind as a residue on the filter paper.
Evaporation
- Evaporation is a method where a liquid turns into its vapour state when heated.
- This method is used to separate a soluble solid from a liquid.
- When we heat the mixture, the liquid evaporates, leaving the solid behind.
- For example, we use evaporation to extract salt from seawater.
- Seawater is collected in shallow beds and allowed to evaporate under the heat of the sun.
- In a few days, the water evaporates, and salt is left behind for use.
Activity 2.7
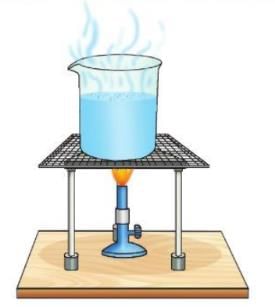
Aim: To separate salt and water by evaporation.
Materials required: Salt solution, a burner, wire mesh, a tripod stand, a beaker.
Procedure:
- Take the salt solution in a beaker.
- Keep the beaker on a tripod stand with a wire mesh and light a burner under it.
- Let the solution boil for some time.
Observation: You will notice that after some time, the water starts evaporating from the beaker. After some more time, all the water evaporates, and only salt is left behind.
Magnetic Separation
- Magnetic separation is used when one part of a mixture is a magnetic substance, like iron.
- We can separate iron from a mixture of iron and sulphur by rolling a magnet over the mixture.
- The iron gets attracted to the magnet and is separated from the mixture.
Sublimation
- Sublimation is a method where a solid turns into vapour directly when heated, without turning into a liquid first.
- This method is used to separate a mixture when one of the substances sublimes on heating.
- For example, we can separate a mixture of ammonium chloride, camphor, naphthalene, or iodine crystals using sublimation.
Activity 2.8
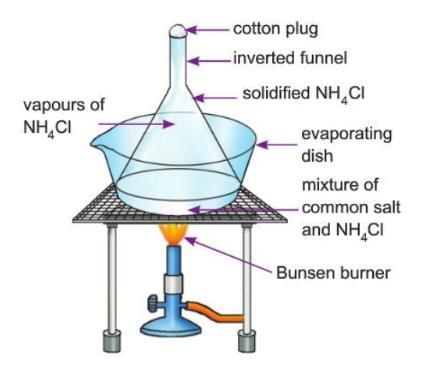
Aim: To separate common salt and ammonium chloride by sublimation.
Materials required: A mixture (solid-solid) of common salt and ammonium chloride (NH₄Cl), an inverted funnel, an evaporating dish, cotton, a Bunsen burner, a tripod stand with mesh.
Procedure:
- Take the mixture of common salt and ammonium chloride (NH₄Cl) in an evaporating dish and place an inverted funnel over it.
- Cover the mouth of the funnel with a piece of paper or cotton.
- Heat the evaporating dish slowly with the help of a tripod stand and burner, and note your observation.
- Remove the burner and allow the system to cool down, and observe the change.
Observations:
- On heating, you will observe vapour (fumes) of NH₄Cl inside the funnel.
- This is the process of sublimation.
- On cooling, you will observe crystals of solid NH₄Cl condense on the cold inner walls of the funnel.
- The common salt is left behind in the evaporating dish.
- The solidified NH₄Cl is called the sublimate.
Some More Methods of Separation
- Handpicking:
- Handpicking is a common method used in daily life to separate mixtures.
- When the amount of mixture is small, we can easily remove impurities like stones and husk by handpicking.
- Winnowing:
- Winnowing is an old farming method to separate lighter particles from heavier ones in a mixture.
- In winnowing, the mixture is dropped from a height, and the wind blows away the lighter particles.
- The heavier particles fall on the ground near the place of winnowing.
- Threshing:
- Threshing is a method used to separate grains from stalks.
- Stalks are dried in the sun, and then the grains are separated from them.
- The stalks are beaten to remove the needed grains.
- Solvent Extraction:
- Solvent extraction is used when one of the solids in a mixture dissolves in a liquid.
- For example, we can separate a mixture of salt and sand using solvent extraction.
- Salt dissolves in water, but sand does not.
- The two can thus be separated.
- Loading:
- Some mixtures have very fine solid particles that settle down very slowly.
- We can make them settle faster by adding special chemicals like alum.
- This process is called loading.
Points To Remember
- Matter is made up of certain basic substances called elements, each of which is made of minute particles called atoms.
- Some atoms can exist on their own, while others are found in combination with atoms of the same or different elements.
- Elements are divided into metals, non-metals, metalloids, and noble gases.
- Most elements are metals, found in the earth's crust, but they are also found in seas and oceans.
- Non-metals are found in abundance in nature.
- Some non-metals are nitrogen, oxygen, krypton, xenon, and radon, while noble gases include boron, silicon, arsenic, and antimony.
- When elements combine with each other in a fixed ratio by mass, they form compounds.
- The smallest unit of a compound is a molecule, which shows all the properties of the compound.
- A molecule is the smallest particle of an element or compound that exists independently and shows all the properties of that substance.
- Elements and compounds have different uses based on their properties.
- In a mixture, the original atoms and molecules stay as they are because no chemical change happens.
- Separation of components in a mixture happens because either the mixture contains harmful or unwanted substances, or we need to obtain pure substances from the mixture.
- Some common methods of separation are sieving, sedimentation and decantation, filtration, evaporation, and magnetic separation.
- In sieving, a mixture is passed through a sieve, where fine particles pass through the sieve, while bigger particles remain on the sieve.
- Sedimentation and decantation are used to separate a solid and a liquid where the solid is heavy and insoluble.
- Filtration is used to separate an insoluble solid and a liquid by passing the mixture through a filter paper.
- Evaporation is used to separate a soluble solid component from a liquid.
- Magnetic separation is used when one of the components of a mixture is a magnetic substance, like iron, and can be attracted by a magnet.
Glossary
- Element: A basic substance that cannot be broken into simpler substances by normal chemical methods.
- Atom: The smallest unit of an element that shows all the properties of that element.
- Molecule: A group of two or more atoms of the same or different elements held together by chemical forces, capable of independent existence.
- Noble (inert) gases: Non-metals that are in a gaseous state and do not react chemically with other elements.
- Metalloids: Elements that have properties of both metals and non-metals.
- Compound: A pure substance formed by the chemical combination of two or more elements in a definite proportion by mass.
- Mixtures: A physical combination of two or more elements or compounds in any proportion.
- Sieving: A method used to separate components of a mixture that differ in size.
- Sedimentation: The process of settling down of suspended insoluble particles at the bottom under the influence of gravity.
- Sediment: The insoluble solid substance that settles down.
- Decantation: Pouring of clear liquid into another beaker without disturbing the sediment.
- Supernatant liquid: The clear liquid over the sediment.
- Residue: The insoluble solid left behind on the filter paper.
- Filtration: A method used to separate an insoluble solid and a liquid.
- Filtrate: The clear liquid that passes through the filter paper.
- Evaporation: A process by which a liquid is converted into its vapour state.
- Magnetic separation: A method used to separate components of a mixture containing a magnetic substance.
- Sublimation: The process by which a solid changes into vapour directly without going into the liquid state on heating.
|
191 videos|265 docs|160 tests
|
FAQs on Elements, Compounds and Mixtures Chapter Notes - Chemistry for EmSAT Achieve
| 1. What are elements and how are they different from compounds? |  |
| 2. Can you explain what the symbols of elements represent? |  |
| 3. What are some common uses of elements and compounds in everyday life? |  |
| 4. What is the difference between atoms and molecules? |  |
| 5. How can I remember the symbols of elements? |  |