Water Chapter Notes | Chemistry Class 6 ICSE PDF Download
Introduction
Water is very important for all living things like humans, animals, and plants. We use water every day for drinking, cooking, bathing, and many other things. This chapter will teach us about what water is, where it comes from, why it is important, and how we can save it. We will also learn about the water cycle, different states of water, and how water behaves when it gets hot or cold.
Occurrence of Water
- Water covers about 70% of the Earth's surface.
- Out of this, 97% of water is in oceans and seas.
- The remaining 3% is fresh water, found in rivers, lakes, ponds, mountains, and glaciers.
- Fresh water in rivers, lakes, and ponds is used for drinking and farming.
- Water is also present in the air as water vapor, clouds, and mist.
- In our body, about 70% is water, which helps us do many things like digest food.
- Fruits, vegetables, and grains we eat also have water in them.
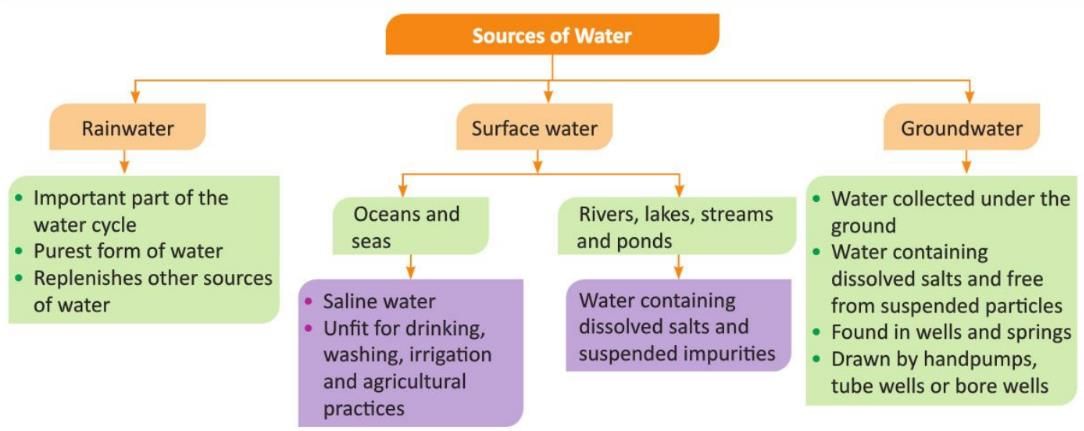
Sources of Water
There are three main sources of water: Rainwater, Surface water, and Groundwater.
Rainwater
- Rainwater is the purest form of water.
- It helps fill up other water sources like rivers and lakes.
- But when it falls, it can get dirty because of dust, nitrogen, sulphur, and other gases in the air due to human activities.
Surface Water
Surface water is the water we see on the Earth's surface. It is found in oceans, seas, rivers, lakes, streams, and ponds.
- Oceans and seas:
- They cover 70% of the Earth's surface.
- Water in oceans has a lot of salt, so it is called saline water.
- The saltiness of sea water is 35 g/kg, which means 35 grams of salt in 1 kg of water.
- Saline water is not good for drinking, farming, or washing.
- Rivers, lakes, streams, and ponds:
- This water comes from rain or melting snow on mountains.
- Water in rivers and lakes flows through hills and plains and gets a little dirty with salts and impurities.
Activity 4.2
Aim: To study the presence of dissolved impurities in natural water.
Materials required: 2 China dishes, some rainwater, some sea water.
Procedure:
- Take rainwater in one China dish and sea water in another China dish.
- Keep both China dishes in the sun and let the water dry up.
Observation: After the water dries, you will see some leftover material.
- In the rainwater dish, there will be very little leftover material.
- In the sea water dish, there will be a lot of leftover material, mostly salt.
Conclusion: This shows that dissolved impurities are different in different water sources. Sea water has more impurities than rainwater.
Groundwater
- Rainwater falls on the ground and goes into the soil.
- This water collects under the ground and is called groundwater or underground water.
- The level of this water under the ground is called the water table.
- The water table changes depending on the place and the season.
- Groundwater is very pure because it passes through many layers of sand, rocks, and soil.
- These layers filter the water and remove dirt and impurities.
- Groundwater also has some dissolved salts in it, but it is still clean.
- We can find groundwater in wells and springs.
- A well is a pool of water made by digging the soil until we reach the water under the ground.
- Under the ground, there is a layer called the impervious layer that does not let water pass through.
- A spring is when groundwater comes out of the ground naturally because of pressure.
- We can also get groundwater using a handpump or a tube well or a bore well.
- A handpump is a tool used to pull groundwater from under the ground.
- A tube well or bore well is a deep hole made in the ground to reach the water.
Activity 4.3
Aim: To check how much dissolved impurities are in natural water.
Materials Required:
- 2 China dishes
- Some rainwater
- Some sea water
Procedure:
- Take some rainwater in one China dish.
- Take some sea water in another China dish.
- Keep both China dishes in the sun.
- Let the water in the dishes evaporate (dry up).
Observation:
- After the water dries up, look at the China dishes.
- The dish with rainwater will have very little or no residue (leftover dirt).
- The dish with sea water will have a lot of residue (leftover dirt).
Conclusion:
- This shows that rainwater has very few dissolved impurities.
- Sea water has a lot of dissolved impurities because the residue is more.
- The amount of impurities in water changes depending on where the water comes from.
States of Water
Water can be in three forms: solid, liquid, and gas.
- Solid state: Water becomes ice or snow when it is very cold (0°C or less).
- Liquid state: Water is a liquid at room temperature, like the water we drink.
- Gaseous state: Water becomes steam or water vapor when it is very hot (100°C or more).

Water Cycle
- The water cycle is the continuous movement of water from the Earth to the sky and back to the Earth.
- Water is found in oceans, seas, rivers, lakes, ponds, and streams.
- Plants also give out water through their leaves in a process called transpiration.
- The heat of the sun makes water from these places turn into water vapor.
- This water vapor goes up into the sky and forms tiny droplets in the air.
- These tiny droplets come together to form clouds.
- When clouds get heavy with water, the droplets become big.
- Then, they fall back to the Earth as rain, hail, sleet, or snow.
- Rainwater goes back to oceans, seas, rivers, lakes, ponds, and streams.
- Some water also goes into the soil and becomes groundwater.
- Plants take up water through their roots, and the water evaporates again from their leaves.
- This whole process keeps repeating, and that is called the water cycle.
Importance of Water Cycle
- The water cycle helps to keep the level of groundwater steady.
- It makes sure there is enough water on Earth.
- However, if there is not enough rain, the groundwater level can go down.
- This can cause a shortage of water for farming and other uses.
- The water cycle also helps to control the climate (weather) of places all over the world.
Change in Density and Volume of Water with Temperature
- Water changes its density (how heavy it is) and volume (how much space it takes) when the temperature changes.
- Normally, when water is heated, it expands (takes up more space) and its density decreases (it becomes lighter).
- When water is cooled, it contracts (takes up less space) and its density increases (it becomes heavier).
- But water behaves differently between 0°C and 4°C.
- When water is cooled from 4°C to 0°C, it expands instead of contracting.
- This means its density decreases, so it becomes lighter than water at 4°C.
- Because of this, ice at 0°C floats on water because it is lighter than liquid water.
- This behavior of water is called the anomalous expansion of water.
- The density of water is highest at 4°C, and this is called the maximum density of water.
- This special property of water helps animals and plants survive in cold places.
- In cold regions, when water in lakes, rivers, or ponds freezes, the ice forms on the top.
- The ice on top protects the water below, so it does not freeze completely.
- This keeps the water liquid underneath, allowing fish and other water animals to live even in very cold weather.
Activity 4.4
Aim: To show that ice is lighter than water.
Materials required: A glass, some water, a few ice cubes.
Procedure:
- Fill half of the glass with water.
- Add a few ice cubes to it.
Observation: The ice cubes float on the water.
Conclusion: This shows that ice is lighter than water because its density is less than water's density.
Activity 4.5
Aim: To show that water expands on freezing.
Materials required: A thick plastic bottle, some water, a marker, a refrigerator.
Procedure:
- Fill half of the plastic bottle with water.
- Mark the water level with a marker.
- Keep the bottle in the freezer.
Observation: After some time, the water level rises above the mark because it freezes into ice.
Conclusion: This shows that water expands when it cools below 4°C and freezes at 0°C.
Note: Do not fill the bottle completely with water because it might break when the water expands and freezes.
Specific Heat Capacity
- Specific heat capacity is the amount of heat needed to raise the temperature of 1 kg of a substance by 1°C.
- Water has a high specific heat capacity of 4186 joule kg⁻¹ °C⁻¹.
- This means water takes a lot of heat to get hot, so it is used as a coolant in car radiators.
Latent Heat of Fusion of Ice
- Latent heat of fusion is the heat needed to change ice to water at 0°C without changing its temperature.
- For ice, this is 334 × 10³ joule kg⁻¹, which means 334,000 joules of heat are needed to melt 1 kg of ice into water at 0°C.
- This is the same as the heat released when 1 kg of water freezes into ice at 0°C.
Latent Heat of Vaporization
- Latent heat of vaporization is the heat needed to change water into steam at 100°C without changing its temperature.
- For water, this is 2.3 × 10⁶ joule kg⁻¹, which means 2,300,000 joules of heat are needed to turn 1 kg of water into steam at 100°C.
- This is the same as the heat released when 1 kg of steam turns back into water at 100°C.
- Because steam has a lot of heat energy, it causes more severe burns than boiling water.
Importance of Water
- Water is very important for all living things to survive.
- It is a big part of the body of all living beings, like humans, animals, and plants.
- For example, about 80% of an elephant’s body is made of water.
- About 30% of a plant’s body weight is water.
Importance of Water for Humans and Animals
- Water helps in digesting food in the stomach.
- Blood, which has water and blood cells, carries food, oxygen, and carbon dioxide in the body.
- Water helps remove waste from the body of humans and animals through sweat and urine.
- Water controls the body temperature in humans and animals.
- Water can hold a lot of heat, so it does not heat up or cool down very fast.
- On hot days, we sweat, and when the sweat evaporates, it takes heat away from our body and cools us down.
- A human loses about 2.5 to 3 liters of water every day through sweat, urine, and breathing.
- So, we should drink about 3 to 4 liters of water every day to make up for this loss.
- We lose about 0.5 liters of water through breathing.
Gain and Loss of Water in Human Beings
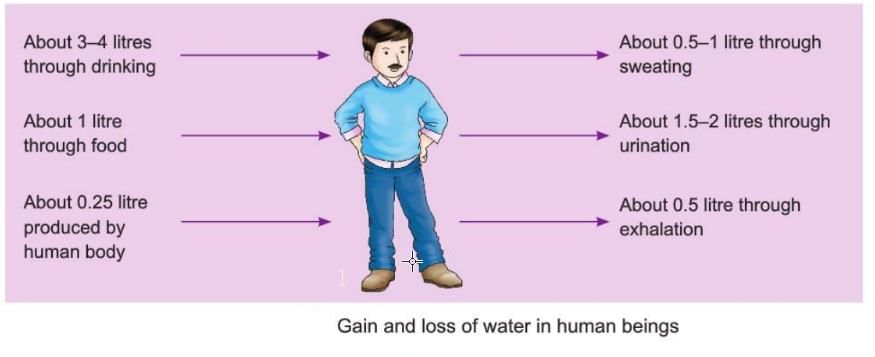
- The image shows how humans gain and lose water every day.
- We gain about 3 to 4 liters of water through drinking.
- We gain about 1 liter of water through the food we eat.
- We gain about 0.25 liters of water produced by the human body.
- We lose about 0.5 liters of water through sweating.
- We lose about 1.5 to 2 liters of water through urine.
- We lose about 0.5 liters of water through breathing.
Importance of Water for Plants
- Plants need a lot of water for photosynthesis, which is how they make their food.
- Water helps carry minerals to different parts of the plant.
- Water is very important for seeds to grow into plants (germination).
- Plants lose water through their leaves during transpiration.
Importance of Water for Aquatic Animals and Plants
- Water is the home for aquatic animals like fish, whales, and dolphins.
- Water plants like Hydrilla, lotus, and water chestnut also need water to live.
Other Uses of Water
- Water is used for bathing, cleaning, and washing things.
- A lot of water is used in farming to grow crops.
- Industries like chemical industries, paper mills, petroleum refineries, fertilizers, steel, and rayon industries use a lot of water.
- Water acts as a coolant in car radiators and powerhouses to cool down machines.
- Water is used to put out fires.
- People in places like Kashmir and Kerala make homes called houseboats and live on water.
- Ships, boats, and sailboats use water to carry people and things from one place to another.
- Water is used for fun activities like swimming, river rafting, boating, and water skiing.
- We get many things from water, like fish, seaweed, oil, natural gas, medicines, minerals, and building materials.
- Rainwater is collected in dams to store water for use in the dry season.
- Water stored in dams is also used to make electricity, which is called hydroelectric power.
Importance of Water
The image shows different uses of water:
- Watering Plants: Water is used to help plants grow.
- Hydropower Generation: Water in dams is used to make electricity.
- Water Transport: Ships and boats use water to travel.
- Fire Extinguishing: Water is used to put out fires.
- River Rafting: Water is used for fun activities like rafting.
- Houseboat: People live in houseboats on water.
Fun Fact: Coolant
- A coolant is a liquid that helps cool down a system by taking heat away from one part to another.
- Water is used as a coolant in many machines, like car engines.
Water—A Universal Solvent
Water can dissolve many things like solids, liquids, and gases. Because water can dissolve so many things, it is called a universal solvent.
Solute, Solvent and Solution
- A substance that gets dissolved in a liquid is called a solute. It is in a smaller amount.
- The liquid that dissolves the solute is called a solvent. It is in a larger amount.
- When a solute and solvent mix together evenly, it is called a solution.
- For example, when we mix sugar (solute) in water (solvent), we get a sugar solution.
- Here are some examples of solutions:
- Common salt and water: Salt is the solute, and water is the solvent.
- Sodium carbonate and water: Sodium carbonate is the solute, and water is the solvent.
- Copper sulphate and water: Copper sulphate is the solute, and water is the solvent.
- Water is not the only solvent. Some things like sand and wood do not dissolve in water.
- Only substances that can dissolve in water are called soluble substances.
- Substances that do not dissolve in water are called insoluble substances.
- The way a solution forms depends on some conditions:
- Stirring: If we stir the solute in the solvent, it dissolves faster because the solute particles mix better. If the solute particles are big, it takes more time to dissolve.
- Temperature: When the temperature of water goes up, most solids and liquids dissolve better. But gases dissolve less in water when the temperature goes up.
Fun Fact
The ability of a solute to dissolve in a solvent is called solubility.
Activity 4.7
Aim: To see how stirring helps make a solution.
Materials needed: 2 beakers, some water, some sugar, a spoon.
Steps:
- Take two beakers and label them as A and B.
- Put the same amount of water in each beaker.
- Add one teaspoon of sugar to both beakers.
- Do not stir the sugar in beaker A.
- Stir the sugar in beaker B.
What you see: The sugar in beaker A will settle at the bottom, but in beaker B, it will dissolve in the water.
Conclusion: Stirring helps the solute dissolve faster in the solvent.
Activity 4.8
Aim: To see how the size of solute particles affects making a solution.
Materials needed: 2 beakers, some water, a lump of common salt, some free-flowing common salt, a spoon.
Steps:
- Take two beakers and label them as A and B.
- Put the same amount of water in each beaker.
- Add a lump of common salt in beaker A and a teaspoon of free-flowing common salt in beaker B. You can also use sugar cubes and powdered sugar.
- Stir both solutions one by one and note the time it takes for the salt to dissolve.
What you see: The salt in beaker B (free-flowing salt) dissolves faster than the salt in beaker A (lump of salt).
Conclusion: Smaller solute particles dissolve faster than bigger ones.
Activity 4.9
Aim: To see how increasing temperature affects making a solution.
Materials needed: Beaker, sugar, spoon, burner.
Steps:
- Take the beaker from the previous activity.
- Add some sugar to it and stir the solution.
- Keep adding sugar until no more sugar can dissolve in the solution.
- Now heat the beaker.
- Add a teaspoon of sugar to it.
What you see: More sugar dissolves in the beaker after heating the solution.
Conclusion: When the temperature goes up, the solubility of a solute increases.
Saturated and Unsaturated Solutions
- In Activity 4.9, we saw that after some time, no more solute can dissolve in the solution at a certain temperature.
- A solution that cannot dissolve any more solute at a particular temperature is called a saturated solution.
- A solution that can still dissolve more solute at a particular temperature is called an unsaturated solution.
- If we add more solute than the solvent can dissolve, it becomes a supersaturated solution.
Fun Fact
The point at which the solution cannot dissolve any more solute is called the saturation point.
Activity 4.10
Aim: To make a saturated solution of salt and water.
Materials needed: A beaker, some water, a spoon, some salt.
Steps:
- Take some water in a beaker.
- Add a teaspoon of salt to it and stir it.
- Keep adding salt until no more salt can dissolve in it.
What you see: After a point, the salt stops dissolving in the water.
Conclusion: The solution becomes saturated, which means no more salt can dissolve at that temperature.
Activity 4.11
Aim: To separate salt from a salt solution by evaporating until the water dries.
Materials needed: A beaker, some water, a spoon, some salt, an evaporating dish, a burner.
Steps:
- Take some water in a beaker and add two teaspoons of salt to it.
- Stir the contents of the beaker to make a salt solution.
- Transfer this salt solution to an evaporating dish.
- Now heat the dish over the flame of a burner.
- Observe what happens after 15–20 minutes.
What you see: After some time, the water starts boiling and turns into water vapour. As more time passes, all the water evaporates, and only salt is left at the bottom of the dish.
Conclusion: This way, we can separate the salt (solute) from the salt solution.
- We can get the solute and solvent back in their original form by evaporating the solution.
- Another way to get the solvent is by distillation, where the water is evaporated and then cooled to form pure water again.
Potable Water
- Water that is safe to drink is called potable water.
- Potable water should have these qualities:
- It should be colourless, without any smell, and clear.
- It should have enough dissolved salts and oxygen in it.
- It should not have harmful chemicals or microorganisms.
- Freshwater and groundwater are good for drinking, but because of more people and factories, freshwater is getting dirty.
- Dirty water can make us sick with diseases called waterborne diseases.
Diseases Caused by Contaminated Water or Waterborne Diseases
- Water in rivers, lakes, etc., should not be used for drinking directly because it may have dirt, waste, or germs from industries, farming, humans, or animals.
- Dirty water can cause diseases like:
- Typhoid: It is caused by bacteria. It affects the intestines and causes high fever for a few days.
- Jaundice: It is caused by the Hepatitis A virus. It affects the liver and causes pain in the stomach and weight loss.
- Dysentery: It is caused by amoeba or bacteria. It affects the intestines, causes mucus in stools, stomach pain, and sometimes anal bleeding.
- Diarrhoea: It causes frequent, loose, or watery stools, making a person weak and tired.
- Gastroenteritis: It is the swelling of the stomach and intestines caused by viruses, bacteria, or their toxins, leading to diarrhoea and vomiting.
- Cholera: It is caused by a bacterium in the small intestine. It causes watery diarrhoea and vomiting.
- We need clean and fresh drinking water to stay safe from these waterborne diseases.
Fun Fact
Infectious diseases that spread mainly through water are called waterborne diseases.
Purification of Water
- Dirty water has mud, sand, germs, and harmful things that need to be removed to make it safe to drink. This process is called purification of water.
- In water treatment plants, water is cleaned before it is sent to our homes.
- We can also clean water at home using some simple methods.
Sedimentation and Decantation
- Sedimentation is when heavy particles in a solution settle down at the bottom.
- It is the first step to clean water in water treatment plants.
- Water from rivers or lakes is put into big tanks and left to stand for some time.
- The heavy dirt particles settle at the bottom, and the clear water stays at the top.
- The clear water at the top is poured out carefully, and this process is called decantation.
Loading
- Loading is a process to make sedimentation faster by adding chemicals.
- A chemical called potash alum is added to the water in the sedimentation tank and left to stand for some time.
- This makes the dirt particles heavier, so they settle down faster at the bottom of the tank.
Filtration
- Filtration is the process of separating a solid from a liquid by passing the mixture through a filter bed or filter paper.
- At home, we pass dirty water through a filter paper to clean it.
- The filter paper has very small holes, so the dirt particles get stuck, and only clean water passes through.
- The dirt left on the filter paper is called residue, and the clean water that passes through is called filtrate.
- In water treatment plants, water from the sedimentation tank is passed through a filter bed.
- A filter bed is made of layers of sand, gravel, and charcoal to clean the water.
Chlorination
- Chlorination is the process of adding chlorine to water to kill harmful bacteria.
- In water supply units of cities, the filtered water is treated with chlorine to kill germs.
- Chlorinated water is stored in a tank for further supply.
Boiling
- Boiling is the easiest and best way to clean water at home.
- When we boil water for 5–10 minutes, it kills most germs that can make us sick.
- This method can be used at home on a small scale.
- Boiling can also be done before using other methods to clean water for extra safety.
Aeration
- Aeration is when air under pressure is blown into filtered water to kill harmful germs.
- This helps make the water cleaner.
Ozonisation
- Nowadays, ozone is used to clean water, and this process is called ozonisation.
- Ozone kills germs in the water to make it safe.
Water Purifiers and RO System
- Water purifiers clean water in three steps:
- In the first step, water passes through a candle filter to remove dirt.
- In the second step, the water goes through activated charcoal to remove more impurities.
- In the third step, the water is treated with ultraviolet radiation to kill germs.
- Reverse osmosis (RO) is another way to clean water.
- In RO, water is forced through a special membrane that removes impurities by applying pressure.
- A lot of dirt is left behind during this process, and the clean water comes out.
- Bottled drinking water is often made using the RO system.
Distillation
- Distilled water is very pure because it has no dissolved salts or minerals, so it tastes plain.
- Distilled water is made by evaporating water and then cooling the vapour to get pure water. This process is called distillation.
- Distilled water is used for making solutions in medical work and in labs.
- It is also used in batteries of inverters and cars.
Importance of Water for Sustenance of Life on Earth
- Water is very important for all living beings to stay alive.
- In our body, water helps in many ways, like moving food and air inside us, keeping our body temperature right, and removing waste.
- Plants need water to make their food through a process called photosynthesis.
- Water helps carry important things like food and air to different parts of plants and animals.
- In our blood, water helps move oxygen and food to all parts of the body.
- Water is also needed by industries to make things and by farmers to grow crops.
- Humans, animals, and plants all need water to live and grow.
Need to Conserve Water
- More people and industries are using a lot of water because the population is growing.
- In some places, people use too much water, which makes it hard to get enough water for everyone.
- We need to save water because we are using more water than what is available.
- Saving water is important so that everyone can have enough water to use.
Steps to Conserve Water
- We should be careful and not waste water while doing things like washing clothes.
- Farmers should use smart ways to water crops, like drip irrigation, which gives water directly to plant roots.
- Dams and reservoirs should be built to store water and use it carefully.
- Water industries should collect and reuse water as much as possible.
- Trees should be planted because they help in making rain, which gives us more water.
- Rainwater harvesting should be done—it means collecting rainwater that falls on buildings and storing it in a deep trench in the ground.
- We can also collect rainwater in buckets and use it for things like watering plants or cleaning.
- We should not waste water while drinking—only take what we need and not leave taps running.
- We should fix leaking taps and pipes quickly to stop water from being wasted.
- Dirty water should be cleaned and reused, and we should not use water for things it’s not needed for.
- We should use less RO purifier water because the unused water from it gets wasted.
- The extra water from RO purifiers should be collected and used for things like cleaning the house.
- Sprinklers should not be used in gardens because they waste water.
- Schools and societies should be encouraged to collect rainwater and use it.
Did You Know?
Rajindra Singh, born in 1959, is a famous water conservationist. He is known as the "Waterman of India." He worked to bring water back to villages in Rajasthan by building over 8600 small dams called johads. His work helped many villages get water again.
Water Pollution
Water pollution happens when harmful things mix with water and make it dirty. Dirty water is not safe for drinking, bathing, or growing plants.
Causes of Water Pollution
- Throwing garbage and sewage into water bodies makes the water dirty.
- Farmers use fertilizers, insecticides, and pesticides, which have harmful things like cyanide and mercury that mix with water.
- Factories throw waste like plastic, paints, drugs, paper, dyes, rubber, and leather into nearby water bodies, making the water dirty.
- Peeing or pooping by humans and animals near water bodies also makes the water dirty.
- Oil spills from ships and oil leakage during drilling make a layer of oil on water, which stops oxygen from reaching plants and animals in the water, causing them to die.
- Waste from nuclear plants thrown into water kills aquatic plants and animals and harms the balance of nature.
Prevention of Water Pollution
- Factories should have water treatment plants to clean dirty water before throwing it into water bodies.
- Sewage, farm waste, and factory waste should be cleaned properly before going into water bodies.
- People should have proper toilets to stop peeing or pooping near water bodies.
- Use things at home that are safe for the environment, like washing powder and cleaning agents that don’t harm nature.
- Farmers should use fewer fertilizers and pesticides, and use eco-friendly fertilizers and biodegradable pesticides instead.
- Keep underground water sources like wells clean and covered so they don’t get dirty.
Floods and Droughts
Flood
- A flood happens when it rains a lot for many days, and rivers overflow with too much water.
- Floods cause a lot of damage in many ways.
- Floods destroy property, kill animals and humans, and damage crops.
- Soil gets washed away because of floods; this is called soil erosion.
- Floods make the water level in dams and rivers rise too much.
- Floods spread diseases and pollution in the water.
Drought
- A drought happens when there is very little rain for a long time, causing a shortage of water.
- Drought affects life in many ways.
- There is not enough food and drinking water during a drought.
- Soil gets washed away because of drought; this is called soil erosion.
- Animals die because there is no water to drink.
- Humans also face health problems because of poor water quality during a drought.
- The groundwater level in lakes, rivers, and wells goes down.
- Plants die, and there is a loss of human lives because of drought.
Points To Remember
- Water is very important for all living things on Earth.
- We can find water in three states: solid, liquid, and gas.
- The three states of water can change into each other by changing temperature.
- Water is an important part of all living things; our body has a lot of water in it.
- How much a substance can dissolve in water depends on things like the size of the substance, how much we stir, and the temperature.
- Based on how much a substance can dissolve, there are three types of solutions: saturated, unsaturated, and supersaturated.
- Potable water should be clean, odorless, and free from harmful chemicals and microorganisms.
- We use methods like boiling, chlorination, and RO systems to clean water for drinking.
- Water pollution happens because of factory waste, farm waste, human and animal waste, nuclear waste, and oil spills.
- We can stop water pollution by cleaning waste, using safe products, and keeping wells clean.
- Floods happen because of heavy rain, causing damage to property, death of animals and humans, damage to crops, soil erosion, rise in water levels, and spread of diseases and pollution.
- Droughts happen because of low rainfall, leading to a lack of food and water, soil erosion, death of animals, health problems, lower groundwater levels, loss of plants, and loss of human lives.
Glossary
- Groundwater: Rainwater that gets collected under the ground
- Water table: The level of water under the ground
- Well water: The pool of water that is obtained by digging the soil till its impervious layer
- Spring water: The water which comes out with pressure in the form of spring from any opening in the earth
- Water cycle: The continuous circulation of water from the earth to the atmosphere and back again
- Solute: A substance that gets dissolved in a liquid
- Solvent: The liquid that dissolves a solute
- Solution: A uniform or homogeneous mixture of the solute and solvent
- Saturated solution: A solution which cannot dissolve any more of the solute at a particular temperature
- Unsaturated solution: A solution which can dissolve more of the solute at a particular temperature
- Supersaturated solution: A solution which contains more solute than could be dissolved in the solvent under normal conditions
- Potable water: An integral component of all living bodies. It is also needed for various other purposes
- Filtrate: The liquid that passes through the filter paper
- Residue: The particulate impurities which remain on the filter paper
- Chlorination: The process of adding chlorine to water for sedimentation
- Water pollution: The contamination of water by unwanted and harmful substances that cause harmful effects to living and non-living things
|
8 videos|46 docs|5 tests
|
FAQs on Water Chapter Notes - Chemistry Class 6 ICSE
| 1. What are the main sources of water on Earth? |  |
| 2. Why is water considered a universal solvent? |  |
| 3. What is potable water and why is it important? |  |
| 4. How does water pollution affect the environment and human health? |  |
| 5. What are the causes and effects of floods and droughts? |  |