Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Mind Map: Study of Compounds – Sulphuric Acid
Mind Map: Study of Compounds – Sulphuric Acid | Chemistry Class 10 ICSE PDF Download
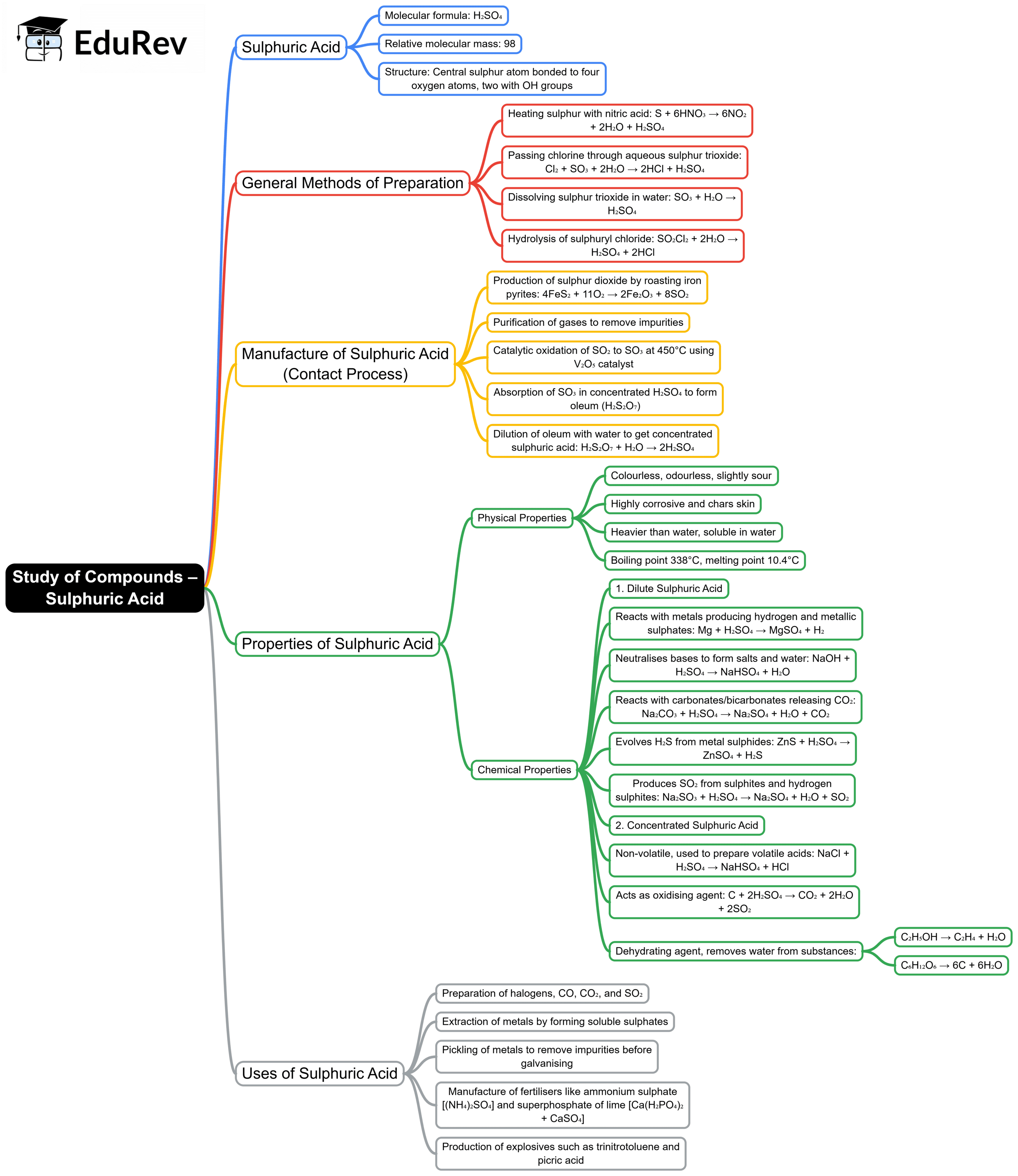
The document Mind Map: Study of Compounds – Sulphuric Acid | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
40 videos|140 docs|14 tests
|
FAQs on Mind Map: Study of Compounds – Sulphuric Acid - Chemistry Class 10 ICSE
| 1. What is sulphuric acid and what are its properties ? |  |
Ans. Sulphuric acid (H₂SO₄) is a strong, colorless, and viscous liquid that is highly soluble in water. It is known for its strong acidic properties and is an excellent dehydrating agent. Its chemical structure consists of two hydrogen atoms, one sulfur atom, and four oxygen atoms. Sulphuric acid has a high boiling point and can cause severe burns upon contact with skin or eyes. It is also a powerful oxidizing agent and can react vigorously with metals and organic materials.
| 2. What are the uses of sulphuric acid in different industries ? |  |
Ans. Sulphuric acid is widely used in various industries. In the manufacturing sector, it is primarily used in the production of fertilizers, especially ammonium sulfate. It is also employed in petroleum refining, chemical synthesis, and the production of explosives. Additionally, sulphuric acid is utilized in battery production, particularly in lead-acid batteries, and in the treatment of wastewater, where it helps in neutralizing alkaline substances.
| 3. How is sulphuric acid produced industrially ? |  |
Ans. Industrially, sulphuric acid is produced using the Contact Process. This involves the oxidation of sulfur dioxide (SO₂) to sulfur trioxide (SO₃) in the presence of a vanadium pentoxide (V₂O₅) catalyst. The sulfur trioxide is then absorbed in water to form sulphuric acid. The overall reaction can be summarized as: 2SO₂ + O₂ → 2SO₃, followed by SO₃ + H₂O → H₂SO₄. This method is efficient and allows for large-scale production.
| 4. What safety precautions should be taken when handling sulphuric acid ? |  |
Ans. When handling sulphuric acid, it is crucial to take several safety precautions. Always wear appropriate personal protective equipment, such as gloves, goggles, and lab coats, to prevent skin and eye contact. Work in a well-ventilated area or use a fume hood to avoid inhaling harmful vapors. In case of spills, neutralize the acid with a suitable base, such as sodium bicarbonate, before cleaning. It is also essential to store sulphuric acid in a properly labeled, corrosion-resistant container away from incompatible materials.
| 5. What are the environmental impacts of sulphuric acid production and use ? |  |
Ans. The production and use of sulphuric acid can have significant environmental impacts. The manufacturing process emits sulfur dioxide (SO₂), which can contribute to air pollution and acid rain. Additionally, improper disposal of sulphuric acid can lead to soil and water contamination, harming aquatic life and disrupting ecosystems. It is important to implement regulations and best practices to minimize these environmental effects, such as using scrubbers to capture emissions and treating waste products before disposal.
Related Searches





















