Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Mind Map: Study of Compounds - Hydrogen Chloride
Mind Map: Study of Compounds - Hydrogen Chloride | Chemistry Class 10 ICSE PDF Download
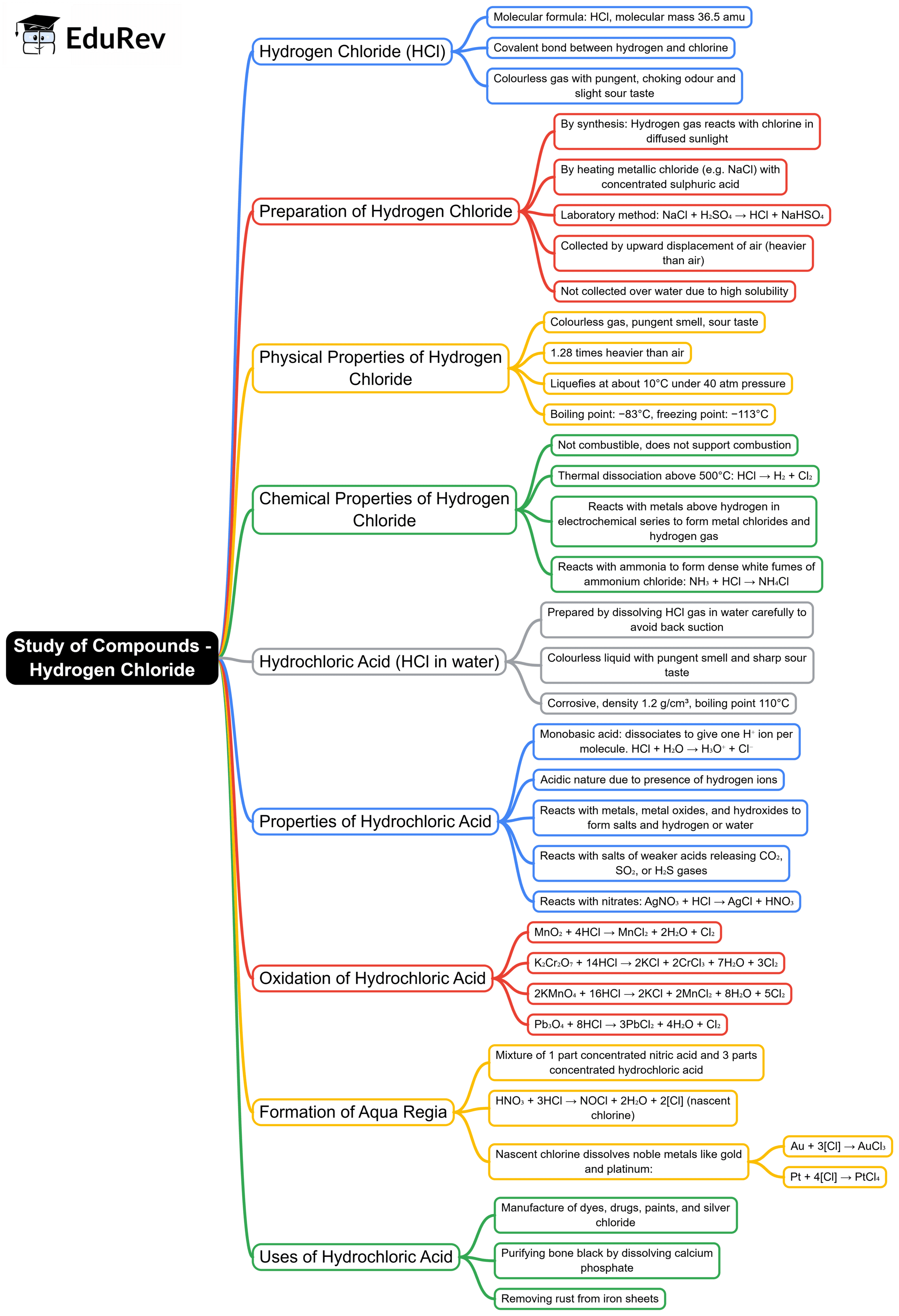
The document Mind Map: Study of Compounds - Hydrogen Chloride | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
40 videos|140 docs|14 tests
|
FAQs on Mind Map: Study of Compounds - Hydrogen Chloride - Chemistry Class 10 ICSE
| 1. What is hydrogen chloride, and how is it formed? |  |
Ans.Hydrogen chloride (HCl) is a colorless gas with a pungent odor. It is formed when hydrogen gas (H₂) reacts with chlorine gas (Cl₂) in a direct combination reaction: H₂(g) + Cl₂(g) → 2HCl(g). This compound dissolves in water to form hydrochloric acid, which is a strong acid commonly used in various industrial processes.
| 2. What are the physical and chemical properties of hydrogen chloride? |  |
Ans.Hydrogen chloride is a gas at room temperature, with a boiling point of -85°C and a melting point of -114°C. It is highly soluble in water, forming hydrochloric acid. Chemically, it is considered a strong acid that completely dissociates in water to produce H⁺ and Cl⁻ ions. It also reacts with bases and certain metals, releasing hydrogen gas.
| 3. What are the uses of hydrogen chloride in everyday life and industry? |  |
Ans.Hydrogen chloride is widely used in various applications. In households, it is found in cleaning products for removing stains and rust. In industries, it is used for the production of chlorides, fertilizers, and dyes, as well as in the food industry for pH control and in the production of hydrochloric acid for metal cleaning and processing.
| 4. How does hydrogen chloride affect human health and the environment? |  |
Ans.Hydrogen chloride is corrosive and can cause severe respiratory problems, skin burns, and eye irritation upon exposure. Inhalation of its vapors can lead to serious health issues. Environmentally, when released into the atmosphere, it can contribute to acid rain, which negatively impacts ecosystems, soil, and water sources.
| 5. What safety precautions should be taken when handling hydrogen chloride? |  |
Ans.When handling hydrogen chloride, it is essential to wear protective equipment, including gloves, goggles, and respiratory protection. Work in a well-ventilated area or use fume hoods to minimize inhalation risks. In case of spills, neutralize with a base, such as sodium bicarbonate, before cleanup, and always follow local regulations for disposal.
Related Searches





















