Worksheet with Solutions: Coordination Compounds | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Section A: Fill In The Blanks |

|
| Section B. Multiple Choice Questions |

|
| Section C: Assertion and Reason |

|
| Section D: Answer the following Questions |

|
Section A: Fill In The Blanks
Q1. Due to dsp² hybridisation, shape of a compound becomes __________.
 View Answer
View Answer 
Answer: square planar
Solution: In dsp² hybridisation, one d-orbital, one s-orbital, and two p-orbitals combine to form four hybrid orbitals arranged in a square planar geometry. This is commonly seen in low-spin d⁸ metal ions like Ni²⁺.
Q2. cis-/trans- isomerism is not possible of __________ structure.
 View Answer
View Answer 
Answer: tetrahedral
Solution: In a tetrahedral structure, all four positions are equivalent, so geometric isomerism like cis-trans is not possible. It requires different spatial arrangements of ligands, which is only possible in square planar or octahedral geometries.
Q3. In metal carbonyls, metal is present in __________ oxidation state.
 View Answer
View Answer 
Answer: zero
Solution: Carbonyl (CO) is a neutral ligand. Since metal carbonyls like Ni(CO)₄ or Fe(CO)₅ are overall neutral compounds, the oxidation state of the metal must be zero.
Q4. As per VBT, hybridisation state of Cu in [CuCl₄]²⁻ is __________.
 View Answer
View Answer 
Answer: sp³
Solution: The complex has a coordination number of 4, and Cl⁻ is a weak field ligand. This leads to sp³ hybridisation using outer orbitals, resulting in a tetrahedral shape.
Q5. Magnetic moment of a high-spin complex is _______ than of a low-spin complex.
 View Answer
View Answer 
Answer: greater
Solution: High-spin complexes have more unpaired electrons than low-spin complexes. Since magnetic moment increases with the number of unpaired electrons, it is greater in high-spin complexes.
Q6. CFSE of an octahedral d⁴ high-spin complex is __________.
 View Answer
View Answer 
Answer: –0.6 Δ₀
Solution: For a high-spin d⁴ configuration:
3 electrons occupy t₂g orbitals (–0.4Δ₀ each)
1 electron occupies e_g orbital (+0.6Δ₀)
CFSE = (3 × –0.4) + (1 × 0.6) = –1.2 + 0.6 = –0.6 Δ₀
Section B. Multiple Choice Questions
Q1. IUPAC name of [Pt(NH3)3 Br (NO2) Cl] Cl isw
(a) triamminechlorodibromidoplatinum (IV) chloride
(b) triamminechloridobromidonitrochloride- platinum (IV) chloride
(c) triamminebromidochloridonitroplatinum (IV) chloride
(d) triamminenitrochlorobromoplatinum (IV) chloride
 View Answer
View Answer 
Answer: c
Explanation: The ligands are named in alphabetical order, regardless of charge:
ammine (NH₃)
bromido (Br⁻)
chlorido (Cl⁻)
nitro (NO₂⁻)
Central metal: platinum (Pt)
Oxidation state of Pt:
Overall complex: [Pt(NH₃)₃Br(NO₂)Cl]⁺
Charge of counter ion (Cl⁻) = –1
So, the cation part has +1 charge
Ligands: NH₃ (neutral), Br⁻ (–1), NO₂⁻ (–1), Cl⁻ (–1) ⇒ Total = –3
So, Pt = +4
Final name: triamminebromidochloridonitroplatinum(IV) chloride
Q2. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5 (ONO)]2+ are called
(a) Ionization isomers
(b) Linkage isomers
(c) Co-ordination isomers
(d) Geometrical isomers
 View Answer
View Answer 
Answer: b
Explanation: NO₂⁻ is an ambidentate ligand: It can coordinate via N atom (nitro form) or O atom (nitrito form). When the same ligand binds through different atoms, the isomers formed are linkage isomers.
Q3. Which of the following has square planar structure?
(a) [NiCl4]2-
(b) [Ni(CO)4]
(c) [Ni(CN)4]2-
(d) None of these
 View Answer
View Answer 
Answer: c
Explanation:
[Ni(CN)₄]²⁻: Ni²⁺ is 3d⁸ configuration. CN⁻ is a strong field ligand, causing pairing.
Hybridisation: dsp²
Shape: square planar
[NiCl₄]²⁻ and [Ni(CO)₄] are tetrahedral because Cl⁻ and CO are weak/moderate field ligands and do not cause pairing (or involve sp³ hybridization).
Q4. Which of the following shall form an octahedral complex?
(a) d1 (low spin)
(b) d8 (high spin)
(c) d6 (low spin)
(d) All of these
 View Answer
View Answer 
Answer: c
Explanation:
In d6 (low spin) complex , two electrons get paired up to make two d orbitals empty. The hybridisation is d2sp3 (octahedral and the complex is low spin complex in the absence of half filled electrons.
Q5. The solution of the complex [Cu(NH3)4] SO4 in water will
(a) give the tests of Cu2+ ion
(b) give the tests of NH3
(c) give the tests of SO42- ions
(d) not give the tests of any of the above
 View Answer
View Answer 
Answer: c
Explanation:
In aqueous solution, [Cu(NH₃)₄]²⁺ is the complex ion and SO₄²⁻ is the counter ion.
Only the counter ion (SO₄²⁻) is free in solution and can be tested using BaCl₂ (white ppt of BaSO₄).
Cu²⁺ is not free, it is complexed with NH₃ — so it doesn't give Cu²⁺ tests.
Similarly, NH₃ is bound — no free NH₃ to detect with Nessler’s reagent.
Section C: Assertion and Reason
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Q1. Assertion : NF3 is a weaker ligand than N(CH3)3.
Reason : NF3 ionizes to give F– ions in aqueous solution.
(a) If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
(b) If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
(c) If the Assertion is correct but Reason is incorrect.
(d) If both the Assertion and Reason are incorrect.
 View Answer
View Answer 
Answer: c
Explanation:
NF₃ is a weaker ligand than N(CH₃)₃ because in NF₃, the nitrogen’s lone pair is strongly pulled toward the highly electronegative fluorine atoms, reducing its ability to donate electrons.
In N(CH₃)₃, the methyl groups are electron-donating and push electron density toward nitrogen, making it a better electron donor (stronger ligand).
However, NF₃ does NOT ionize to give F⁻ ions in water. It's a covalent compound and remains mostly undissociated.
Q2. Assertion : [Fe(CN)6]3– is weakly paramagnetic while [Fe(CN)6]4– is diamagnetic.
Reason : [Fe(CN)6]3– has +3 oxidation state while [Fe(CN)6]4– has +2 oxidation state
(a) If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
(b) If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
(c) If the Assertion is correct but Reason is incorrect.
(d) If both the Assertion and Reason are incorrect.
 View Answer
View Answer 
Answer: b
Explanation:
[Fe(CN)₆]³⁻: Fe³⁺ has 3d⁵ configuration. CN⁻ is a strong field ligand, so pairing occurs, but still one unpaired electron remains ⇒ weakly paramagnetic.
[Fe(CN)₆]⁴⁻: Fe²⁺ has 3d⁶ configuration. CN⁻ causes full pairing ⇒ diamagnetic.
The oxidation states mentioned are correct, but they do not explain the magnetic behavior directly — the nature of the ligand (CN⁻) and crystal field splitting do.
Q3. Assertion : [Ti(H2O)6]3+ is coloured while [Sc(H2O)6]3+ is colourless.
Reason : d-d transition is not possible in [Sc(H2O)6]3+
(a) If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
(b) If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
(c) If the Assertion is correct but Reason is incorrect.
(d) If both the Assertion and Reason are incorrect.
 View Answer
View Answer 
Answer: a
Explanation:
[Ti(H₂O)₆]³⁺ has Ti³⁺ (3d¹) ⇒ d–d transition possible ⇒ coloured.
[Sc(H₂O)₆]³⁺ has Sc³⁺ (3d⁰) ⇒ no d-electrons ⇒ no d–d transitions ⇒ colourless.
So, both statements are correct, and the reason correctly explains the assertion.
Q4. Assertion (A): Linkage isomerism arises in coordination compounds containing ambidentate ligand.
Reason (R): Ambidentate ligand has two different donor atoms.
(a) If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
(b) If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
(c) If the Assertion is correct but Reason is incorrect.
(d) If both the Assertion and Reason are incorrect.
 View Answer
View Answer 
Answer: a
Explanation:
Linkage isomerism occurs when a ligand can coordinate to the metal through two different atoms, e.g., NO₂⁻ can bind via N or O.
These are ambidentate ligands, and their nature explains linkage isomerism.
Q5. Assertion (A): Complexes of the types MX₆ and MX₅L (where X and L represent unidentate ligands) do not exhibit geometrical isomerism.
Reason (R): Complexes with a coordination number of 6 do not display geometrical isomerism.
(a) If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
(b) If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
(c) If the Assertion is correct but Reason is incorrect.
(d) If both the Assertion and Reason are incorrect.
 View Answer
View Answer 
Answer: c
Explanation:
MX₆: All ligands are the same ⇒ No geometrical isomerism.
MX₅L: Only one ligand is different ⇒ Again, no isomerism possible. So assertion is correct.
BUT, the reason is incorrect. Coordination number 6 (e.g., in octahedral complexes like [Co(NH₃)₄Cl₂]⁺) can show geometrical isomerism (cis-trans forms).
Section D: Answer the following Questions
Q1. [Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3- is weakly paramagnetic. Explain. (At. no. Fe = 26) (Comptt. All India 2012)
 View Answer
View Answer 
Answer: In both the cases, Fe is in oxidation state +3. Outer electronic configuration of Fe+3 is :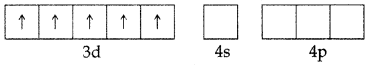
In the presence of CN–, the 3d electrons pair up leaving only one unpaired electron. The hybridisation involved is d2sp3 forming inner orbital complex which is weakly paramagnetic. In the presence of H2O (a weak ligand), 3d electrons do not pair up. The hybridisation involved is sp3d2 forming an outer orbital complex. As it contains five unpaired electrons so it is strongly paramagnetic.
Q2. Explain why [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex. (At. no. Co = 27, Ni = 28)
 View Answer
View Answer 
Answer: In [Co(NH3)6]3+, the d-electrons of Co3+ ([Ar]3d6 45°) get paired leaving behind two empty d-orbital and undergo d2sp3 hybridization and hence inner orbital complex, while in [Ni(NH3)6]2+ the d-electrons of Ni2+ ([Ar]3d8 45°) do not pair up and use outer 4d subshell hence outer orbital complex.
Q3. (i) Write down the IUPAC name of the following complex :
[Cr(NH3)2CI3(en)]Cl (en = ethylenediamine)
(ii) Write the formula for the following complex : Pentaamminenitrito-o-Cobalt (III)
 View Answer
View Answer 
Answer: (i) [Cr(NH3)2Cl3(en)]Cl
IUPAC name : Diammine dichlorido ethylenediamine chromium (III) chloride.
(ii) [Co(NH3)5(ONO)]2+
Q4. For the complex [Fe(en)2Cl2], Cl, (en = ethylene diamine), identify
(i) the oxidation number of iron,
(ii) the hybrid orbitals and the shape of the complex,
(iii) the magnetic behaviour of the complex,
(iv) the number of geometrical isomers,
(v) whether there is an optical isomer also, and
(vi) name of the complex. (At. no. of Fe = 26)
 View Answer
View Answer 
Answer:
(i) [Fe(en)2Cl2] Cl or x + 0 + 2 (-1) + (-1) = 0
x + (- 3) = 0 or x = + 3
∴ Oxidation number of iron, x = + 3
(ii) The complex has two bidentate ligands and two monodentate ligands. Therefore, the coordination number is 6 and hybridization will be d2sp3 and shape will be octahedral.
(iii) In the complex 26Fe3+ = 3d5 4s0 4p0
Due to presence of one unpaired electrons in d orbitals the complex is paramagnetic.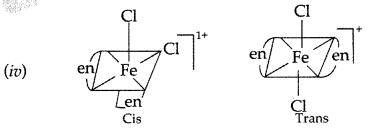 The number of geometrical isomers are two.
The number of geometrical isomers are two.
(v) In coordination complex of [Fe(en)2Cl2] Cl, only cis-isomer shows optical isomerism.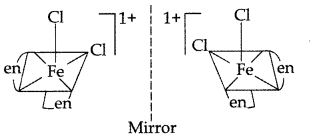
Q5. Compare the following complexes with respect to their shape, magnetic behaviour and the hybrid orbitals involved :
(i) [CoF4]2-
(ii) [Cr(H2O)2(C2O4)2]–
(iii) [Ni(CO)4] (Atomic number : Co = 27, Cr = 24, Ni = 28)
 View Answer
View Answer 
Answer:
(i) [COF4]2_ : Tetrafluorido cobalt (III) ion
Coordination number = 4 Shape = Tetrahedral Hybridisation = sp3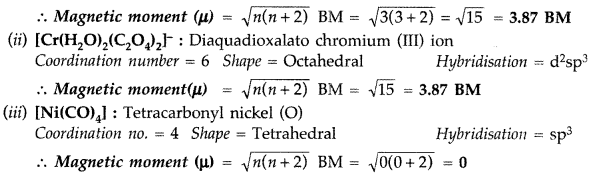
Q6. Giving a suitable example for each, explain the following :
(i) Crystal field splitting
(ii) Linkage isomerism
(iii) Ambidentate ligand
 View Answer
View Answer 
Answer:
(i) Crystal field splitting: It is the splitting of the degenerate energy levels due to the presence of ligands. When ligand approaches a transition metal ion, the degenerate d-orbitals split into two sets, one with lower energy and the other with higher energy. This is known as crystal field splitting and the difference between the lower energy set and higher energy set is known as crystal field splitting energy (CFSE)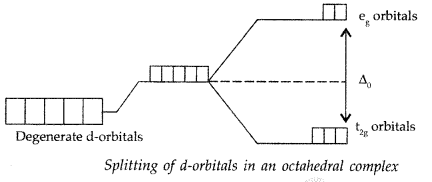 Example : 3d5 of Mn2+
Example : 3d5 of Mn2+
(ii) Linkage isomerism: When more than one atom in an ambidentate ligand is linked with central metal ion to form two types of complexes, then the formed isomers are called linkage isomers and the phenomenon is called linkage isomerism.
[Cr(H2O)5(NCS)]2+ Pentaaquathiocyanate chromium (III) ion
[Cr(H2O)5(NCS)]2+
Pentaaquaisothiocyanate chromium (III) ion
(iii) Ambidentate ligand: The monodentate ligands with more than one coordinating atoms is known as ambidentate ligand. Monodentate ligands have only one atom capable of binding to a central metal atom or ion. For example, the nitrate ion NO2– can bind to the central metal atom/ion at either the nitrogen atom or one of the oxygen atoms.
Example : — SCN thiocyanate, — NCS isothiocyanate
Q7. Compare the following complexes with respect to structural shapes of units, magnetic behaviour and hybrid orbitals involved in units : [Co(NH3)6]+3, [Cr(NH3)6]3+, Ni(CO)4
(At. nos. : Co = 27, Cr = 24, Ni = 28)
 View Answer
View Answer 
Answer: (i) [Co(NH3)6]+3 → Octahedral shape, d2sp3 hybridisation, diamagnetic
Formation of [Co(NH2)6]+3 → oxidation state of Co is +3.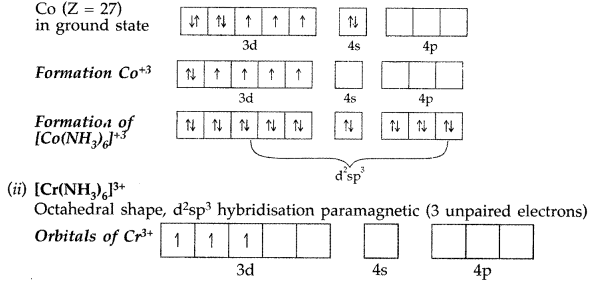
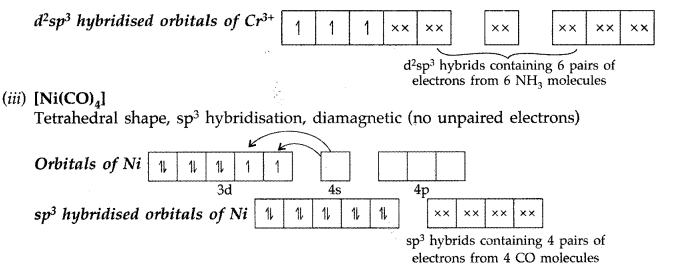
Q8. Explain the following cases giving appropriate reasons :
(i) Nickel does not form low spin octahedral complexes.
(ii) The n-complexes are known for the transition metals only.
 View Answer
View Answer 
Answer:
(i) The electronic configuration of Ni is [Ar] 3d8 4s2 which shows that it can only form two types of complexes i.e. square planar (dsp2) in presence of strong ligand and tetrahedral (sp3) in presence of weak ligand. There are four empty orbitals in Ni while octahedral complexes require six empty orbitals.
(ii) Due to presence of empty d-orbitals in transition metals, they can accept electron pairs from ligands containing π electrons and hence can form ic-bonding complexes.
Example : ligands like C5H5, C6H6 etc.
(iii) Due to greater magnitude of Δ0, CO produces strong fields which cause more splitting of d-orbitals and moreover it is also able to form π bond due to back bonding.
Q9. Write the state of hybridization, the shape and the magnetic behaviour of the following complex entities :
(i) [Cr(NH3)4Cl2]Cl
(ii) [Co(en)3]Cl3
(iii) K2[Ni(CN)4]
 View Answer
View Answer 
Answer:
(i) [Cr(NH3)s4Cl2]Cl :
Hybridization : d2sp3
Shape : Octahedral
Magnetic behaviour: Paramagnetic
(ii) [Co(en)3] Cl3 :
Hybridization : d2sp3
Shape : Octahedral
Magnetic behaviour : Diamagnetic
(iii) K3[Ni(CN)4] :
Hybridization ; dsp2
Shape : Square planar
Magnetic behaviour: Diamagnetic
Q10. Write the name, the structure and the magnetic behaviour of each one of the following complexes :
(i) [Pt(NH3)2Cl(NO2)]
(ii) [Co(NH3)4Cl2]Cl
(iii) Ni(CO)4 (Atmos. Co = 27, Ni = 28, Pt = 78)
 View Answer
View Answer 
Answer: (i) [Pt(NH3)2Cl(NO2)]
Name : Diamine chloridonitroplatinum II
Structure :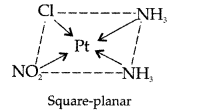 Magnetic behaviour: paramagnetic
Magnetic behaviour: paramagnetic
(ii) [Co(NH3)4Cl2]Cl
Name : Tetraamminedichloridocobalt (III) chloride
Structure : octahedral
Magnetic behaviour : diamagnetic
(iii) Ni(CO)4
Name : Tetracarbonylnickel (O)
Structure : tetrahedral
Magnetic behaviour : diamagnetic.
|
75 videos|278 docs|78 tests
|
FAQs on Worksheet with Solutions: Coordination Compounds - Chemistry Class 12 - NEET
| 1. What are coordination compounds and how are they formed? |  |
| 2. What is the significance of the oxidation state of the metal in coordination compounds? |  |
| 3. How do you determine the coordination number of a metal in a complex? |  |
| 4. What are some common types of ligands found in coordination compounds? |  |
| 5. How do coordination compounds differ from ionic compounds? |  |















