Elements, Compounds and Mixtures Chapter Notes | Chemistry Class 7 ICSE PDF Download
Introduction
Everything around us is made of matter. The basic building blocks of matter are called atoms. When atoms of the same kind come together, they form an element. When atoms of different kinds join in fixed amounts, they form a compound. In this chapter, you will learn about elements, compounds, and mixtures, how they are different, and how mixtures can be separated using simple methods.
Elements
- An element is a pure substance made up of only one kind of atom. It is the simplest form of matter and cannot be broken down into anything simpler by physical or chemical methods.
- Examples of elements include hydrogen, carbon, oxygen, and aluminium.
- The smallest unit of an element is called an atom, and it takes part in chemical reactions. Each element has its own unique type of atom, which gives it special properties.
- Scientists have discovered 118 elements so far. Out of these, 98 are found in nature, and the rest are man-made in laboratories.
- Elements are grouped into metals, non-metals, metalloids, and noble gases based on their properties.
Metals
- Examples of Metals include copper, silver, gold, aluminium, iron, and platinum.
- Most metals are hard solids with high strength. However, there are exceptions: mercury and gallium are liquids at room temperature, while sodium and potassium are soft metals.
- Metals have a shiny surface called metallic lustre.
- Many metals are ductile (can be drawn into wires) and malleable (can be hammered into sheets). However, zinc is an exception as it is brittle.
- Metals are excellent conductors of heat and electricity.
- Metals are also sonorous, meaning they produce sound when struck.
- Most metals have high melting and boiling points. However, potassium, sodium, and calcium have relatively low melting and boiling points.
Non-Metals
- Examples: Sulfur, carbon, and phosphorus are solid non-metals, while oxygen, nitrogen, chlorine, and hydrogen are gaseous non-metals.
- State: Most non-metals are either soft solids or gases at room temperature. The only exception is bromine, which is a liquid.
- Surface Appearance: Non-metals typically have a dull and non-shiny surface. However, iodine and graphite are exceptions as they have a shiny appearance.
- Brittleness: Solid non-metals are usually brittle and can easily break into a powder.
- Conductivity: Non-metals are generally poor conductors of heat and electricity. The notable exception is graphite, which is capable of conducting both heat and electricity.
- Sound Production: Non-metals do not produce sound when they are struck.
- Melting and Boiling Points: Non-metals have low melting and boiling points.
Metalloids
- Metalloids exhibit a combination of properties found in both metals and non-metals.
- Examples of metalloids include germanium, arsenic, silicon, boron, antimony, and tellurium.
- Due to their unique properties, metalloids are commonly used as electrical semiconductors.
Noble Gases
- Noble gases, also known as inert gases, are non-metals that exist in a gaseous state.
- These gases are characterised by their low reactivity, as they do not readily engage in chemical reactions with other elements or compounds.
- Examples of noble gases include helium, neon, argon, xenon, krypton, and radon.
- Noble gases are present in trace amounts in the Earth's atmosphere.
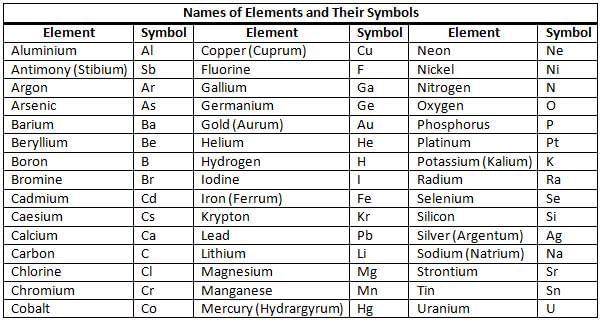
Names and Symbols of Elements
- Element names come from various languages like English, German, Latin, and Greek, as well as from places and the scientists who discovered them.
- Jöns Jacob Berzelius played a crucial role in standardising the modern system for element symbols by creating a system that uses letters to represent elements.
- The International Union of Pure and Applied Chemistry (IUPAC) assigns a unique symbol to each element, usually consisting of the first letter of the element's name, such as H for hydrogen and O for oxygen.
- If two elements have the same first letter, the second letter is included, like C for carbon and Cl for chlorine.
- The first letter of a symbol is always uppercase, and the second letter is lowercase. Some symbols come from Latin or Greek names, such as Pb for lead, which comes from 'plumbum'.
- Examples of elements and symbols: Aluminium (Al), Copper (Cu), Gold (Au), Hydrogen (H), Oxygen (O), Sodium (Na).
Compounds
A compound is a pure substance that is formed when two or more elements chemically combine in a definite proportion by mass. In other words, a compound is formed when atoms or molecules of different elements combine.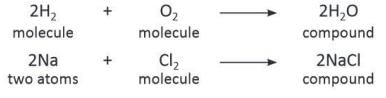
Characteristics of a Compound
- Compounds are created when two or more elements are chemically bonded together.
- The properties of a compound are different from those of the individual elements it contains. For example, while hydrogen and oxygen are gases, water is a liquid.
- Each compound has a fixed proportion of its elements.
- During chemical reactions involving compounds, energy is either absorbed or released.
- Compounds are represented by chemical formulas, similar to how elements are shown with symbols.
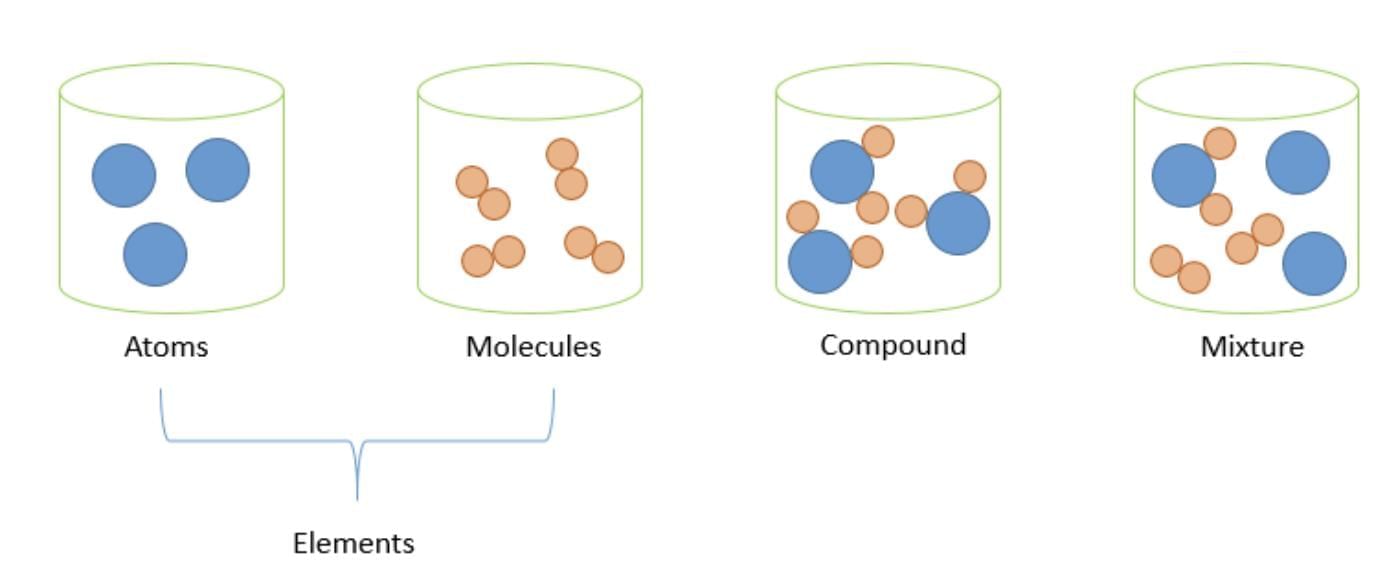
Atoms
- An atom is the smallest part of an element that can take part in a chemical reaction.
- Atoms usually cannot exist alone but join with other atoms to form molecules.
- Examples of molecules are H₂, O₂, P₄, S₈, H₂O, NH₃, and NaCl.
Molecules
- Definition: A molecule is a group of atoms bonded together, acting as a single unit with its own specific properties.
- Composition: Molecules can consist of atoms of the same element (e.g., O₂) or atoms of different elements (e.g., H₂O).
- Independence: Some molecules can exist independently under normal conditions.
Activity 3.1
Aim: To show that the smallest pieces of aluminium or zinc still have the same properties as aluminium or zinc.
Materials: Aluminium or zinc.
Procedure:
- Take a small piece of aluminium or zinc.
- Grind it into small pieces.
Observation: The small pieces still show the properties of aluminium or zinc, but they cannot be divided further. These smallest pieces are called atoms.
Conclusion: The smallest pieces of aluminium or zinc still have the properties of the original material.
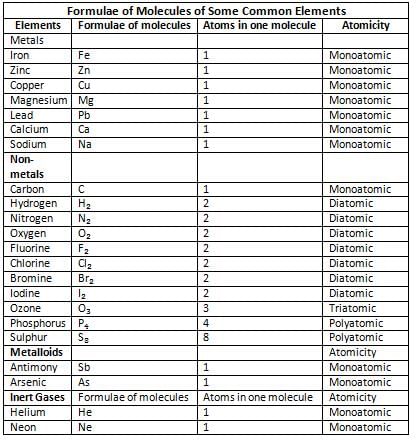
Atomicity
- Atomicity is all about how many atoms are packed into a molecule of a particular element.
- Depending on the number of atoms, molecules are divided into four groups: monoatomic (one atom), diatomic (two atoms), triatomic (three atoms), and polyatomic (more than three atoms).
Monoatomic Molecules

- Monoatomic molecules have only one atom in their molecule.
- Examples are helium (He), neon (Ne), argon (Ar), sodium (Na), and iron (Fe).
- The symbol and molecular formula for these elements are the same, like He for helium and Ar for argon.
Fun Fact
Some metals and metalloids also have only one atom in their molecules, but they are not considered true monoatomic molecules because they form a large structure, like a crystal, in their natural state.
Diatomic Molecules
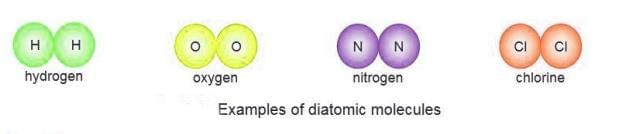
- Diatomic molecules have two atoms of the same element in their molecule.
- Examples are hydrogen (H₂), oxygen (O₂), chlorine (Cl₂), and nitrogen (N₂).
- These molecules are called diatomic because they have two atoms.
Triatomic Molecules
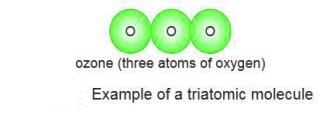
- Triatomic molecules have three atoms in their molecule.
- An example is ozone (O₃), which is a form of oxygen.
- These molecules are called triatomic because they have three atoms.
Polyatomic Molecules
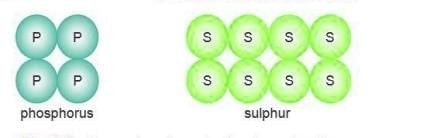
- Polyatomic molecules are made up of more than two atoms and play a crucial role in various chemical reactions and compounds.
- Examples of polyatomic molecules include phosphorus (P₄) and sulfur (S₈). In these examples, phosphorus consists of 4 atoms, while sulfur is composed of 8 atoms.
- The term "polyatomic" comes from the fact that these molecules are formed by multiple atoms.
Molecular Formula
- A molecular formula shows the number of atoms of each element in one molecule of a compound.
- It is also called a chemical formula.
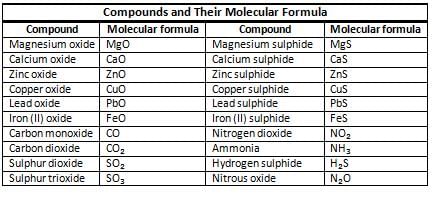
Significance of a Formula
- A chemical formula provides crucial information about a compound, including:
- The elements present: It indicates which elements are included in the compound.
- The number of atoms: It specifies how many atoms of each element are found in the compound.
- Mass calculation: The formula helps in calculating the mass of a single molecule of the compound.
For instance, in NH₃ (ammonia), the formula indicates that there is one nitrogen atom and three hydrogen atoms in each molecule.
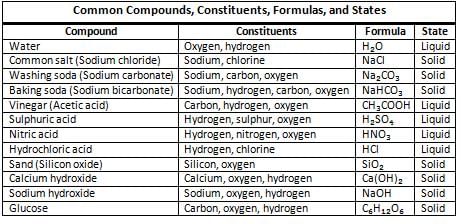
Mixtures
- Mixtures are formed when two or more substances, which can be either elements or compounds, are combined.
- In a mixture, the individual components retain their unique properties and can be separated using simple methods.
- The process of creating a mixture does not involve any chemical change.
- Examples of mixtures include: Air, rocks, wood, lemonade, and milk.
Characteristics of a Mixture
- Varying Amounts: The components of a mixture can be combined in any proportion. For example, the amounts of carbon dioxide and water vapour in the air can change depending on environmental conditions.
- No Chemical Reaction: There is no chemical reaction between the parts of a mixture. They retain their individual properties.
- Easy Separation: The components are held together loosely, making them easy to separate. For instance, sand can be separated from water using filtration.
- Retained Properties: The components maintain their own properties in a mixture. For example, air can support combustion because of the oxygen it contains.
- No Fixed Melting or Boiling Point: Mixtures do not have a specific melting or boiling point.
- Variable Properties: Mixtures do not have fixed physical or chemical properties, as these can vary based on the components present.
- Minimal Energy Change: While mixtures typically do not involve significant chemical reactions, the energy changes that occur are minor compared to those in chemical processes.
- Examples: Common examples of mixtures include salt and water, which illustrate the concept of solubility.
Types of Mixtures
Mixtures are of two types:- homogeneous
- heterogeneous.

Homogeneous Mixture
- A homogeneous mixture is the same throughout and has the same properties everywhere.
Example: Sugar solution is equally sweet everywhere; seawater, alloys like steel, bronze, brass, and solder are also homogeneous. - An alloy is a metal mixture made by mixing two or more metals, or a metal and a non-metal, to get better properties.
Heterogeneous Mixture
- A heterogeneous mixture, on the other hand, varies in composition and has different properties in different parts of the mixture. Examples of heterogeneous mixtures include sand and water, oil and water, dyes, soil, blood, sand and salt, suspensions, and emulsions.
- Emulsion: An emulsion is a specific type of heterogeneous mixture where tiny droplets of one liquid are dispersed in another liquid, such as oil in water.
- Suspension: Suspension is a heterogeneous mixture containing small solid particles floating in a liquid, like sand in water.
Activity 3.2
Aim: To make a homogeneous mixture.
Materials: Sugar, water, a glass, a spoon.
Procedure:
- Add one spoonful of sugar to a glass of water.
- Stir well to dissolve the sugar.
Observation: A homogeneous solution is formed.
Conclusion: Water is the solvent, and sugar is the solute in this solution.
Activity 3.3
Aim: To make a heterogeneous mixture.
Materials: A glass bottle, water, cooking oil (like groundnut or mustard oil).
Procedure:
- Mix oil and water in a glass bottle.
- Observation: Oil, being lighter, forms a layer above water.
- Shake the mixture well.
- Observation: The layers mix, and the mixture looks milky (this is called an emulsion).
- Let the mixture sit for some time.
Observation: Oil and water separate again.
Conclusion: Oil and water form a heterogeneous mixture.
- Deforestation reduces the soil’s ability to hold plants.
- We should plant more trees to improve soil conditions.
Formation of Mixtures
Mixtures can be made by mixing solids, liquids, and gases.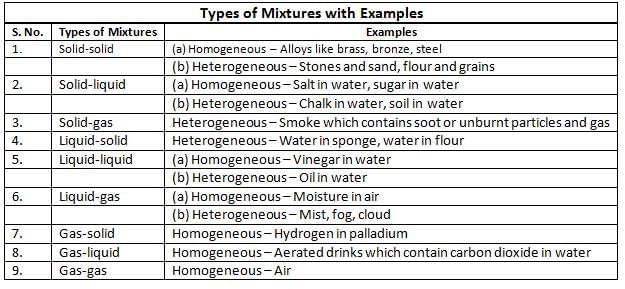
Activity 3.4
Aim: To show that a compound’s properties are different from a mixture’s properties.
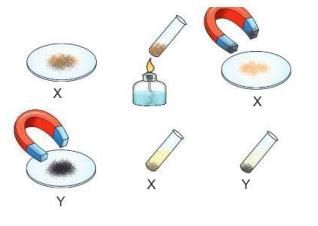
Materials: Iron filings, sulphur, magnet, liquid carbon disulphide, 3 test tubes, 2 watch glasses, a burner.
Procedure:
- Mix iron filings and sulphur, put some on a watch glass, and label it as sample X.
- Put the rest in a test tube and heat it.
Observation: A black mass (iron sulphide) forms, which is a compound.
Procedure
- Place the iron sulphide on another watch glass and label it as sample Y.
- Roll a magnet over samples X and Y.
- Add carbon disulphide to both samples in separate test tubes.
Observation: In sample X, sulphur dissolves in carbon disulphide, but iron does not. In sample Y, nothing dissolves.
Conclusion: A mixture (sample X) shows the properties of its parts (iron and sulphur), but a compound (sample Y) has different properties. Iron sulphide is formed in a fixed ratio (7:4) and does not act like iron or sulphur.
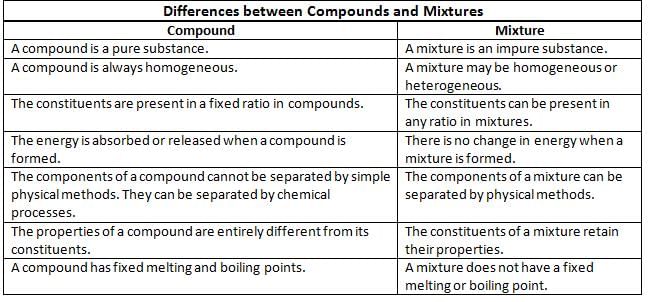
Differences between Compounds and Mixtures
- Compounds are considered pure substances, while mixtures are classified as impure substances.
- Compounds are always homogeneous, meaning they have a uniform composition throughout. In contrast, mixtures can be either homogeneous (uniform composition) or heterogeneous (distinct and separate components).
- The components in a compound are present in a fixed ratio, whereas the components in a mixture can be present in any ratio.
- The formation of a compound involves an energy change, either by absorbing or releasing energy. On the other hand, the formation of a mixture does not involve any significant energy change.
- Compounds can only be separated into their individual components using chemical methods, while mixtures can be separated using physical methods.
- The properties of a compound are different from the properties of its individual components. In a mixture, the components retain their own individual properties.
- Compounds have specific melting and boiling points, while mixtures do not have fixed melting or boiling points.
Methods of Separating Mixtures
Mixtures are impure substances, so we need to learn how to separate them into their parts.Need for Separation of Mixtures
- We separate mixtures to remove harmful or unwanted substances.
- It helps to get useful substances (e.g., removing impurities from food before cooking).
- It is done to get pure substances for making medicines or for experiments in labs.
Solid-Solid Mixtures
We can use sublimation to separate the parts of a solid-solid mixture.Sublimation
- Sublimation is a process where a solid changes directly into a gas without passing through the liquid state.
- This method is used to separate mixtures when one of the components can sublime.
- Substances like ammonium chloride, camphor, and naphthalene are examples of materials that can sublime.
Activity 3.5
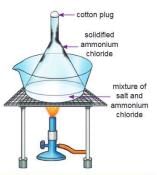
Aim: To separate a mixture of common salt and ammonium chloride.
Materials:A China dish, a funnel, a wire gauze, a tripod stand, common salt, ammonium chloride, cotton wool, a Bunsen burner.Procedure:
- Take the mixture of salt and ammonium chloride in a China dish.
- Cover the dish with an upside-down glass funnel.
- Close the end of the funnel with cotton wool.
- Heat the mixture gently.
Observation: White fumes come out when heating. These fumes turn back into solid ammonium chloride on the sides of the funnel as white powder. This powder is called sublimate and can be collected as pure ammonium chloride. Salt stays in the dish because it does not sublime.
Conclusion: Ammonium chloride sublimes on heating, but salt does not, so we can separate them using sublimation.
Solid-Liquid Mixtures
We can use methods like evaporation and distillation to separate a solid-liquid mixture.Evaporation
- Evaporation is used to separate a solid from a liquid in a homogeneous mixture.
- Evaporation means a liquid turns into a vapour when heated, leaving the solid behind. The process gets faster when the temperature is higher.
- Example: We can separate salt from a salt-water mixture using evaporation.
Activity 3.6
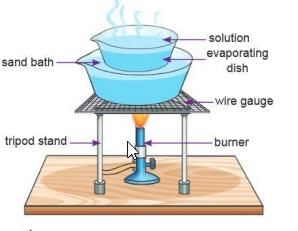
Aim: To separate salt from a mixture of salt and water.
Materials: An evaporating dish, common salt, water, a Bunsen burner, a tripod stand, a wire gauze.Procedure:
- Take the salt-water mixture in an evaporating dish.
- Heat the mixture.
Observation: When heated, the water turns into vapor and goes away. White salt particles are left in the dish after all the water evaporates.
Conclusion: We can get salt from a salt-water mixture using evaporation.
Distillation
- Distillation is used to get the liquid part from a solid-liquid homogeneous mixture.
- Distillation means heating a liquid to turn it into vapour, then cooling the vapour to turn it back into a liquid.
- It is done using a special setup called a distillation flask.
- The mixture is heated in the distillation flask, and the liquid turns into vapour, leaving the solid behind.
- The vapour goes through a condenser, which cools it down, and the liquid is collected in a flask.
- Distilled water, used by doctors and in labs, is made by distillation.
Activity 3.7
Aim: To get pure water by distillation.
Materials: A distillation flask, a condenser, a Bunsen burner, a salt-water mixture, a conical flask.
Procedure:
- Set up the apparatus as shown in the figure.
- Take the salt-water mixture in the distillation flask.
- Heat the mixture.
Observation: When heated, the water turns into vapor. The vapor cools in the condenser and turns back into liquid, which is collected in the conical flask. Salt stays in the distillation flask.
Conclusion: Distillation can separate both the solid (salt) and pure liquid (water) from the mixture.
Liquid-Liquid Mixtures
- Liquid-liquid mixtures can be of two types: miscible liquids and immiscible liquids.
- Miscible liquids mix completely with each other and form one layer (e.g., alcohol and water, milk and water).
- Immiscible liquids do not mix and form separate layers (e.g., oil and water).
Fractional Distillation
- Fractional distillation is used to separate miscible liquids that have different boiling points.
- It works because different liquids boil at different temperatures.
- The liquid with the lower boiling point turns into vapour first and is collected after cooling.
Example: A mixture of alcohol and water can be separated by fractional distillation. - Alcohol has a lower boiling point (78°C) than water, so it evaporates first.
- This method works when the boiling points of the liquids differ by 25°C or more.
- Fractional distillation is used to get petrol, diesel, and kerosene from crude petroleum.
Activity 3.8
Aim: To separate a mixture of alcohol and water.
Materials: A mixture of alcohol and water, a distillation flask, a Bunsen burner, a thermometer, a fractionating column, a condenser, a conical flask.
Procedure:
- Set up the apparatus as shown in the figure.
- Take the alcohol-water mixture in the distillation flask.
- Heat the mixture.
Observation: Alcohol, with a lower boiling point (78°C), turns into vapor first. The vapor cools in the condenser and is collected as liquid alcohol in the conical flask. Water, with a higher boiling point, stays in the distillation flask.
Conclusion: Fractional distillation can separate alcohol and water.
Using a Separating Funnel
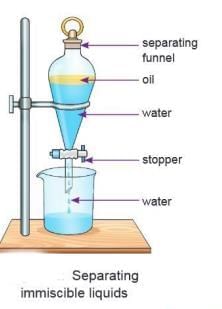
- A separating funnel is used to separate immiscible liquids that form layers.
- The heavier liquid settles at the bottom, and the lighter liquid stays on top.
Example: A mixture of mustard oil and water can be separated using a separating funnel. - The mixture is poured into the funnel and left to stand for some time.
- Two layers form: oil (lighter) on top and water (heavier) at the bottom.
- The stopper is opened to let the heavier liquid (water) come out first and be collected in a container.
- The stopper is closed once all the water is out, leaving the oil in the funnel.
- This method can also be used for mixtures like oil and water, petrol and water, or carbon tetrachloride and water.
Gas-Liquid Mixtures
Gases dissolved in a liquid come out when the liquid is heated.- Example: When water is boiled, the dissolved gases escape, and the water becomes tasteless.
Combining Methods of Separation
We sometimes use more than one method to separate a mixture with more than two parts.Separating salt, sand, and grain
- Sieving: Separates grain from the mixture to keep it dry.
- Filtration: Mix salt and sand with water, then filter to separate sand; salt stays in the water.
- Evaporation: Heat the salt-water mixture to evaporate the water and get the salt.
Separating iron filings, sand, and iodine
- Magnetic separation: Uses a magnet to remove iron filings from sand and iodine.
- Sublimation: Heats the mixture to turn iodine into gas, leaving sand behind.
Chromatography
- Chromatography is a method used to separate components of a mixture that are very similar to each other, either in a liquid or gas form.
- The separation process relies on how different components of the mixture interact with a fixed material, a process known as adsorption.
- Chromatography consists of two phases:
Mobile Phase: This is a liquid or solvent that carries the mixture.
Stationary Phase: This is a solid material, such as paper, that remains fixed in place. - Example of Paper Chromatography: In paper chromatography, the paper serves as the stationary phase, while the solvent, like water, acts as the mobile phase.
Paper Chromatography
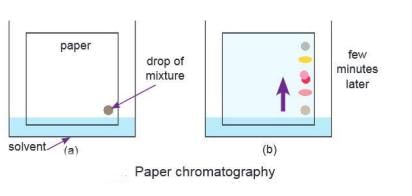
- Paper chromatography is a technique used to separate and analyse different parts of a mixture.
- It involves the use of special paper, known as the stationary phase, and a liquid called the mobile phase.
- The process begins by placing the mixture on the paper, which is then immersed in the solvent.
- As the solvent moves up the paper through a process called capillary action, it carries the mixture along with it.
- Components of the mixture that have a greater affinity for the solvent travel faster, while those that adhere more strongly to the paper move at a slower pace.
- This difference in movement leads to the separation of the mixture's components.
Uses of Paper Chromatography
- Paper chromatography is a valuable tool for identifying the individual components of a mixture.
- It is commonly used in laboratories to detect unknown organic and inorganic substances.
- The technique is particularly effective in separating colored mixtures, such as pigments found in inks or plant materials.
- Additionally, paper chromatography is used to separate amino acids, which are the building blocks of proteins.
- In the field of biochemistry, it aids in the analysis of various biochemical reactions.
- The method is also employed to check for impurities in food and beverages, ensuring safety and quality.
- In medicine, paper chromatography is used to identify hormones and drugs in biological samples.
Activity 3.9
Aim: To separate different dyes in a mixture of ink.
Materials: High-quality filter paper strip (Whatman Grade 1), ink mixture, water with 10% alcohol, a glass jar.
Procedure:
- Take a strip of paper and draw a line with a pencil.
- Mark a point in the center to place the ink mixture.
- Hang the paper strip inside the glass jar.
- Fill the jar with the water-alcohol solvent, keeping the level below the line.
- Let it sit for some time.
Observation: The solvent moves up the paper and dissolves the ink. Different colors in the ink move at different distances.
Conclusion: The solvent is the mobile phase, and the paper is the stationary phase. Each color moves differently because of how much it sticks to the paper or dissolves in the solvent. The paper with the separated colors is called a chromatogram.
Glossary
- Pure substances: Substances made of atoms or molecules with a fixed structure.
- Impure substances: Substances made by mixing two or more things without changing their chemical nature.
- Element: The simplest pure substance made of only one type of atom.
- Atom: The smallest part of an element that takes part in a chemical reaction.
- Compound: A pure substance made when two or more elements join chemically in a fixed ratio.
- Molecule: A group of atoms held together, acting as one unit with its own properties.
- Atomicity: The number of atoms in a molecule of an element.
- Monoatomic molecules: Molecules with one atom of an element.
- Diatomic molecules: Molecules with two atoms of an element.
- Triatomic molecules: Molecules with three atoms of an element.
- Polyatomic molecules: Molecules with more than three atoms of an element.
- Molecular formula: A symbol showing the number of atoms of each element in a compound’s molecule.
- Mixtures: Combinations of two or more elements or compounds.
- Homogeneous mixture: A mixture that is the same throughout with the same properties everywhere.
- Solution: A homogeneous mixture where a solute dissolves in a solvent.
- Solvent: The substance in which a solute dissolves.
- Heterogeneous mixture: A mixture that is not the same throughout and has different properties in different parts.
- Sublimation: The process where a solid turns directly into a gas without becoming a liquid.
- Evaporation: The process by which a liquid turns into vapour.
- Distillation: The process of heating a liquid to vapour, then cooling it back to liquid to separate it.
- Miscible liquids: Liquids that mix completely with each other in all amounts.
- Immiscible liquids: Liquids that do not mix and form separate layers.
- Fractional distillation: The process of separating miscible liquids with different boiling points.
- Chromatography: A method to separate a mixture based on how well parts stick to a surface.
- Paper chromatography: A type of chromatography using paper and a solvent to separate mixtures.
|
33 videos|58 docs|7 tests
|
FAQs on Elements, Compounds and Mixtures Chapter Notes - Chemistry Class 7 ICSE
| 1. What are elements, and how are they classified in chemistry? |  |
| 2. What is the difference between compounds and mixtures? |  |
| 3. What is atomicity, and how does it relate to molecules? |  |
| 4. How are molecular formulas different from empirical formulas? |  |
| 5. Can you provide examples of common mixtures and their characteristics? |  |