Heat Chapter Notes | Science for Grade 4 PDF Download
| Table of contents |

|
| Introduction |

|
| Heat Energy and Its General Effects |

|
| Temperature and Its Measurement |

|
| Thermal Expansion and Its Applications |

|
| Transmission of Heat |

|
Introduction
Heat refers to the transfer of thermal energy between systems or objects with different temperatures. In this chapter, we will delve into the impact of heat energy on our everyday lives. We will learn about:
- The nature of heat and how it alters the temperature of objects.
- The expansion and contraction of materials due to heat.
- The transformation of states in objects, such as ice melting into water or water evaporating into steam.
Heat Energy and Its General Effects
Heat Energy
Heat Energy refers to the energy that causes objects to feel hot or cold when touched. This energy is transferred from one object to another, creating the sensation of temperature.
- When you touch an ice cube, it feels very cold because heat from your hand is transferred to the ice. This transfer of heat is what makes the ice feel cold to the touch.
- Similarly, when you touch warm water, it feels warm because heat from the water is moving to your hand. This is an example of heat energy flowing from a hotter object (the water) to a cooler one (your hand).
- Boiling water feels extremely hot because it transfers a large amount of heat to your hand almost instantly. This is why we use terms like cold, lukewarm, warm, hot, boiling hot, steaming hot, and burning hot to describe how something feels.
- We can gauge how hot or cold something is by touching it, but this method is subjective and may not provide accurate measurements. For example, when we touch an ice cube, it feels very cold because heat moves quickly from our hand to the ice. However, when we touch tap water, it might feel cold simply because our body is warmer than the water.
- Heat is a form of energy that flows from a hotter object to a colder one, creating the sensations of hot and cold.
General Effects of Heat Energy
Heat energy can change things in many ways, and these changes happen in both materials and living things. Here are the effects of heat energy:
(a) Heat energy brings about a change in temperature
- When we give heat to a solid, liquid, or gas, its temperature goes up.
- For example, if we heat a solid, its temperature rises.
- But when we take heat away from a solid, liquid, or gas, its temperature falls.
- For example, when we cool something, its temperature goes down.
(b) Heat energy brings about a change in dimensions
- Applying heat to a solid, liquid, or gas makes it expand, changing its size.
- For instance, when a solid is heated, its length, area, and volume increase.
- Heating a liquid causes its volume to increase.
- Heating a gas also leads to an increase in volume.
- Cooling a solid, liquid, or gas makes it contract, resulting in a smaller size.
- This means the length, area, and volume of a solid decrease when cooled.
- The volume of a liquid or gas also decreases when cooled.
- The size increase from heating is known as thermal expansion.
- The size decrease when cooled is referred to as contraction.
(c) Heat energy brings about a change in state
Heat can change the state of a material, which means it can turn a solid into a liquid, a liquid into a gas, or a gas back into a liquid or solid.
- Melting: When we heat a solid, like ice, it turns into a liquid, like water, at a certain temperature called the melting point. For example, ice melts at 0°C and turns into water because it takes in heat energy.
- Fusion or melting: This is the process where a solid turns into a liquid by taking in heat energy at a fixed temperature. For example, ice changes to water at 0°C, which is its melting point.
- Fusion point or melting point: This is the fixed temperature at which a solid changes into a liquid. For example, the melting point of ice is 0°C, which means ice turns into water at this temperature.
- Freezing or solidification: When we cool a liquid, like water, it turns into a solid, like ice, at a fixed temperature called the freezing point. For example, water turns into ice at 0°C by giving out heat energy.
- Freezing point or solidification point: This is the fixed temperature at which a liquid turns into a solid. For example, 0°C is the freezing point of water, which means water turns into ice at this temperature.
- Vaporisation or boiling: When we heat a liquid, like water, it turns into a gas, like steam, at a fixed temperature by taking in heat energy. For example, water turns into steam at 100°C by taking in heat energy.
- Boiling point or vaporisation point: This is the fixed temperature at which a liquid turns into a gas. For example, the boiling point of water is 100°C, which means water turns into steam at this temperature.
- Condensation: When we cool a gas, like steam, it turns back into a liquid, like water, at a fixed temperature by giving out heat energy. For example, steam at 100°C turns into water at 100°C by giving out heat energy.
- Condensation point: This is the fixed temperature at which a gas turns into a liquid. For example, steam turns into water at 100°C, which is its condensation point.
- Evaporation: This is when a liquid turns into its gas form at any temperature below its boiling point. For example, water in a bucket slowly turns into water vapour even at room temperature because of evaporation.
- Sublimation: Sublimation is when a solid directly changes into a gas on heating, or a gas directly changes into a solid on cooling. For example, solid substances like naphthalene or camphor change directly into a gas when heated, without becoming a liquid. When this gas is cooled, it turns back into a solid without becoming a liquid. The solid form of these substances is called the sublimate.
(d) Heat energy brings about chemical change
- Heat energy can trigger chemical reactions, changing one substance into another by breaking and forming new bonds.
- For instance, heating calcium carbonate (marble) breaks it down into calcium oxide and carbon dioxide.
- Another example is heating potassium chlorate, which decomposes into potassium chloride and oxygen gas.
- Heating materials like charcoal, paper, or wood in the air causes them to burn as they react with oxygen.
- This combustion produces carbon dioxide gas, water vapour, and a significant amount of heat and light energy.
- The process of burning in air or oxygen, releasing heat and light energy, is called combustion.
- Substances that ignite easily in air or oxygen are termed flammable or combustible substances.
- Examples of flammable substances include paper, wood, charcoal, coal, petrol, diesel, kerosene oil, cooking gas (LPG), alcohol, and natural gas (CNG).
- Substances that do not ignite easily in air or oxygen are known as non-flammable or incombustible substances.
- Examples of incombustible substances include metals like iron, gold, and silver, as well as glass, mica, asbestos, sand, and stone.
Sources of Heat Energy
- The Sun: The Sun produces a huge amount of heat and light energy through the process of fusion in its core. Solar panels can convert this sunlight into electricity or heat, allowing us to harness the Sun's energy here on Earth.
- Earth: The interior of our planet is extremely hot, and in volcanic regions, this heat can escape in the form of hot water or steam. This hot water or steam is used to generate heat energy in various locations.
- Wood and Biomass: When wood is burned, it produces heat energy. Similarly, biomass materials such as dried leaves, cow dung, and any plant or animal waste also generate heat when they are burned. In India, for example, cow dung gas plants convert cow dung into methane gas, which is a valuable source of heat energy.
- Coal and Petroleum: Coal and petroleum are formed from the remains of ancient plants and animals that were buried under the Earth's surface millions of years ago. When coal or petroleum is burned, it releases heat energy. These materials are called fossil fuels because of their origins.
Units of Heat
- In the CGS system, heat energy is measured in calories (cal).
- In the SI system, heat energy is measured in joules (J).
- 1 calorie (cal) is the heat energy required to raise the temperature of 1 gram of water by 1°C under standard conditions.
- 1 kilocalorie (kcal) equals 1000 calories.
- 1 calorie is equal to 4.2 joules.
Temperature and Its Measurement
- In our daily lives, we often come across objects that are either cold, like ice, or warm, like water. When we touch these objects, we can feel their temperature. For instance, ice feels cold to the touch, while warm water feels hot.
- Similarly, when a person has a fever, their body feels hot, and it's important to measure just how hot it is. This is where the concept of temperature comes in. Temperature is a way of telling us how hot or cold something is.
- To measure temperature, we use a special tool called a thermometer. A thermometer helps us find out the exact temperature of an object, whether it's something as simple as ice or as important as checking a fever.
Thermometer
- A thermometer is a device used to measure the temperature of different objects.
Description of Thermometer
- A widely used laboratory thermometer is known as a Celsius thermometer.
- It functions based on the principle of thermal expansion, which means liquids expand when heated.
- The thermometer comprises a thin glass tube called a capillary tube, with a small bulb at one end.
- This bulb contains a liquid metal known as mercury.
- The other end of the capillary tube is sealed, and there is no air inside it.
- The capillary tube is encased within a thicker protective glass tube called the stem.
- The stem is marked with graduations ranging from -10°C to 110°C to indicate temperature.
- These markings are known as degrees.
- Two important points are indicated on the stem: the lower standard point and the upper standard point.
- The lower standard point, also called the lower fixed point, is 0°C, which is the melting point of pure ice under standard atmospheric pressure of 76 cm of mercury.
- The upper standard point, or upper fixed point, is 100°C, which is the boiling point of pure water at the same atmospheric pressure of 76 cm of mercury.
Qualities of a Good Thermometric Liquid
A good thermometric liquid should have these qualities:
- Minimal Heat Absorption: The liquid should absorb only a small amount of heat from the object being measured. This ensures that the object's temperature does not change significantly during the measurement process.
- Uniform Expansion: The liquid should expand uniformly to allow for the marking of a linear scale on the thermometer's stem. This uniformity is crucial for accurate readings.
- Wide Temperature Range: The liquid should be capable of measuring a broad range of temperatures, including very low freezing points and high boiling points. This versatility is essential for measuring the temperatures of various substances.
- Non-Adhesive Properties: The liquid should not stick to the sides of the capillary tube. This property is important for ensuring accurate and consistent readings.
- Visibility: The liquid should be opaque, coloured, or shiny, making it easily visible within the capillary tube. This visibility aids in taking precise measurements.
- Availability: The liquid should be readily available in its pure form. For example, mercury is often used because it is available in a pure state and possesses the necessary properties for a thermometric liquid.
Reasons for Using Mercury in Thermometers
- Mercury expands with very little heat, making it ideal for measuring the temperature of living organisms.
- Mercury can measure high temperatures up to 357°C, which is suitable for various high-temperature applications.
- Mercury is capable of measuring low temperatures down to -39°C, which is useful for items like ice cream.
- Mercury does not stick to the sides of the capillary tube, ensuring accurate temperature readings.
- The opaque and shiny appearance of mercury makes it look like a fine thread in the capillary tube, facilitating easy visibility.
However, due to the health risks associated with mercury exposure, its use in thermometers is being replaced by safer alternatives.
Reasons for Using Alcohol in Thermometers
- Some alcohol thermometers can accurately measure temperatures as low as -100°C, making them ideal for extremely cold regions like the Arctic and Antarctic.
- However, alcohol thermometers are not reliable for temperatures above -39°C.
- Alcohol expands more than mercury for the same increase in temperature, which leads to more precise temperature readings.
- Additionally, the bright colours of alcohol make it easily visible within the glass capillary tube, further enhancing its effectiveness in thermometers.
Temperature Scales and Their Inter-Conversion
There are three scales to measure temperature: Celsius, Kelvin, and Fahrenheit.
1. Celsius Scale
- The Celsius scale is commonly used for everyday temperature readings, while Kelvin is the official SI unit for temperature.
- The lower point on the Celsius scale is 0°C, which represents the melting point of ice, and the upper point is 100°C, the boiling point of water.
- This range is divided into 100 equal parts, with each part representing 1°C.
- The Celsius scale is widely used around the world for daily temperature measurements.
2. Kelvin Scale
- The Kelvin scale is mainly used in scientific contexts and is the SI unit for temperature.
- It was named after the scientist Lord Kelvin.
- In the Kelvin scale, 0°C is equivalent to 273 K, and 100°C is equivalent to 373 K.
- To convert from Celsius to Kelvin, you add 273 to the Celsius temperature. For example, 30°C is equal to 303 K (30 + 273).
- To convert from Kelvin to Celsius, you subtract 273 from the Kelvin temperature. For example, 353 K is equal to 80°C (353 - 273).
3. Fahrenheit Scale
- The Fahrenheit scale was developed by Daniel Gabriel Fahrenheit.
- In this scale, the lower point is 32°F, which is the melting point of ice, and the upper point is 212°F, the boiling point of water.
- The range between these two points is divided into 180 equal parts.
- In the Fahrenheit scale, 0°F is 32 degrees below the freezing point of water.
Conversion of Celsius, Fahrenheit and Kelvin Scales
- To convert Celsius to Fahrenheit, use this formula: (C / 5) = (F - 32) / 9.
- Another way to write this is: C / 5 = (F - 32) / 9 or F = (9C / 5) + 32.
- To convert Celsius to Kelvin, use this formula: Temperature in Kelvin = Temperature in °C + 273.
Thermal Expansion and Its Applications
Thermal Expansion
- Thermal Expansion: When materials, whether they are solids, liquids, or gases, are heated, they generally expand, and when they are cooled, they contract. This phenomenon is known as thermal expansion and contraction.
- Thermal expansion is crucial for understanding how materials behave when their temperature changes. When heated, materials can expand in terms of length, width, or thickness.
There are three main types of thermal expansion:
- Linear Expansion: This type of expansion occurs when the length of a solid increases due to heat. For example, a metal rod will become longer when it is heated.
- Superficial Expansion: Superficial expansion happens when the area of a solid increase with heat. For instance, a heated metal plate will expand in both length and width.
- Cubical Expansion: This type of expansion involves an increase in the volume of a solid, liquid, or gas when heated. For example, water expands when it is heated, and so does the air in a balloon.
Gravesande’s Ring and Ball Experiment
- In this experiment, we use a metal ball and a metal ring to observe the effects of heating and cooling on solids.
- The ball is connected to a metal stand with a chain, and the ring is also placed on the stand. The ball and the ring are nearly the same size, allowing the ball to pass through the ring easily at the beginning.
- We start by heating the ring using a Bunsen burner for about two minutes. After heating, when we try to pass the ball through the ring, it does not fit. This is because the ring expands when heated, while the ball remains the same size.
- Once the ball cools down to room temperature, it can pass through the ring again. This experiment demonstrates that most solids shrink when they cool down.
To Prove Equal Lengths of Different Solids Expand by Different Amounts when Heated Equally
- Bimetallic Strip Experiment: We take two strips of brass and iron that have the same dimensions and join them with a wooden handle to make a bimetallic strip.
- Heating the Strip: We hold the handle and use a Bunsen flame to heat one end of the bimetallic strip for 5 minutes.
- Observing the Bend: We see that the strip bends, with brass on the outer side and iron on the inner side.
- Understanding the Cause: This occurs because brass expands more than iron when heated to the same temperature within a given range of temperatures.
- Result of Unequal Expansion: This bending results from the unequal expansion of the two metals.
Practical Applications of Thermal Expansion of Solids
Opening of a Jammed Lid of a Bottle
- When a bottle lid becomes stuck, it can be difficult to open.
- By placing the lid in hot water, the heat helps to expand the lid slightly, making it easier to unscrew.
- This is because the metal or plastic of the lid expands with heat, reducing the tightness of its grip.
Mounting of Iron Rim on Wooden Cart Wheels
- An iron rim, which is initially smaller than the wooden wheel, is used for added durability.
- To fit the rim onto the wheel, it is heated until it becomes red hot.
- When heated, the iron rim expands, allowing it to fit over the wooden wheel.
- After placing the rim on the wheel, cold water is poured over it. This cooling process causes the rim to shrink, tightening its grip on the wheel.
- Even on hot summer days, pouring cold water helps maintain the secure fit of the iron rim on the wooden wheel.
Fitting an Axle into a Wheel
- The axle is first cooled in liquid nitrogen at extremely low temperatures, reaching about -200°C. This cooling process causes the axle to shrink, making it easier to fit into the wheel.
- After the axle is fitted into the wheel, it is heated again. This heating causes the axle to expand, creating a snug fit within the wheel.
Laying of Rail Tracks
- Rail tracks are constructed from steel, a material that expands when heated and contracts when cooled.
- If the rails are fixed too tightly, there won't be sufficient space for them to expand during the hot summer months. This lack of space can lead to the rails bending and potentially causing a derailment.
- To avoid this issue, small gaps are intentionally left between the rails during the summer to accommodate their expansion.
- Even though these gaps widen during the winter months, the rails remain aligned, which helps reduce the risk of derailment.
Mounting of Bridges
- The girders of a bridge are not fixed to the pillars because they expand when heated and shrink when cooled.
- If they are fixed, they can break the pillars when they expand or shrink.
- We mount the girders on rollers, leaving a small space for them to expand or shrink.
- We also leave small spaces behind the walls to allow for expansion and contraction.
Laying of Telephone and Electric Transmission Wires
- Metal wires expand in summer and sag down, and they can break if they are too tight.
- In winter, the wires shrink, and if they are too loose, they can break because of the tightness.
- We keep the wires slightly loose in summer so they can expand without breaking.
- In winter, the wires are kept tight so they can shrink without sagging too much.
- This allows for expansion in summer and contraction in winter.
Laying of Cement Floor
- In summer, cement floors expand, and if there isn't enough room for this expansion, they can crack.
- To prevent this, we lay the cement floor in small blocks with tiny spaces between them.
- These gaps allow the blocks to expand without causing any damage.
Breaking a Thick Glass Tumbler
- When hot water is poured into a thick glass tumbler, it can break because glass does not transfer heat quickly.
- The inner surface of the glass heats up and expands more than the outer surface, causing strain due to this uneven expansion.
- This strain leads to cracks in the glass.
- Similarly, if ice is placed in the glass tumbler, it can also crack.
- This is because the inner surface shrinks more rapidly than the outer surface, creating uneven contraction and resulting in cracks.
Bimetallic Strip and Fire Alarm
- A bimetallic strip is made by joining two different metals, such as brass and invar, at several points to prevent them from sliding.
- At room temperature, the bimetallic strip remains straight.
- When heated, the brass expands more than the invar, causing the strip to bend with brass on the outside and invar on the inside.
- Conversely, when cooled below room temperature, the brass shrinks more than the invar, resulting in the strip bending with invar on the outside and brass on the inside.
Automatic Fire Alarm
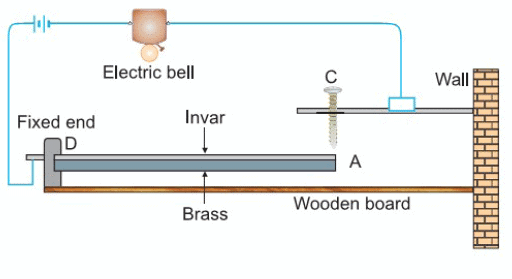
- We use a bimetallic strip made of Invar and brass in a fire alarm.
- The invar-brass strip is fixed at one end (D) on a wooden board, and the other end (A) is free to move up and down.
- We connect an electric circuit to a bell.
- We keep a contact screw (C) made of brass about 1 mm above the free end (A) of the strip.
- When there is a fire, the bimetallic strip gets heated and bends.
- Since brass expands more than invar, the free end (A) rises and touches the contact screw (C).
- When A touches C, the electric circuit is completed, and the bell starts ringing to warn of the fire.
Thermal Expansion of Liquids
- Liquids do not have a fixed shape, but they have a fixed volume.
- Liquids take the shape of the container they are in, but their volume stays the same.
- When we heat a liquid, its volume increases because the molecules move faster and need more space.
- This increase in volume when a liquid is heated is called thermal expansion of liquids.
Thermal Expansion of Gases
- Gases do not have a fixed shape or volume in an open container.
- In a closed container, gases have a fixed volume.
- When we heat a gas in a closed container, its molecules move faster and push harder against the container.
- This makes the volume of the gas increase, which is called thermal expansion of gases.
- The expansion of gases is much greater than solids or liquids.
Transmission of Heat
Introduction
Heat is a form of energy that makes things warm or hot. In this chapter, we will learn how heat moves from one place to another. There are three main ways heat can travel: conduction, convection, and radiation. Conduction happens when heat moves through solids without the particles moving much. Convection is when heat moves through liquids and gases because the particles move around. Radiation is when heat travels directly from a hot object to a cold one without needing anything in between. We will also explore how some materials let heat pass easily, called conductors, while others do not, called insulators. Let’s understand these ideas step by step!
Conduction
- Heat travels through solids by a process called conduction.
- In conduction, the particles of the solid do not move from their places but pass heat energy to nearby particles.
- Heat moves from the hot end to the cold end of a solid object.
Good Conductors of Heat
- Good conductors are materials that let heat pass through them easily.
- All metals, like copper, iron, silver, and aluminium, are good conductors of heat.
- Silver is the best conductor of heat, followed by copper, aluminium, gold, and iron.
Practical Applications of Good Conductors
- Cooking pots and pans are made of metals because metals quickly take in heat and transfer it to the food.
- Mercury, a liquid metal, is used in thermometers because it is a good conductor and quickly shows temperature changes.
- Copper tubes are used in car radiators because copper easily takes heat away from the hot water coming from the engine.
- Air conditioners and refrigerators have copper cooling coils because copper quickly takes heat away.
- Soldering rods are made of copper because copper easily conducts heat to melt the solder.
Bad Conductors of Heat or Heat Insulators
- Bad conductors, also called insulators, do not let heat pass through them easily.
- Materials like glass, mica, cotton, wool, asbestos, felt, air, and polyurethane foam (PUF) are bad conductors of heat.
- Liquids (except mercury) and gases are also bad conductors of heat.
Practical Applications of Heat Insulators
- We wear woollen clothes in winter because wool is a bad conductor and keeps body heat from escaping, making us feel warm.
- New quilts are warmer than old ones because they have more air trapped inside, and air is a poor conductor of heat.
- Two thin woollen blankets are warmer than one thick blanket because the air trapped between them acts as an insulator.
- Birds puff up their feathers in winter to trap air, which acts as an insulator and keeps them warm.
- Eskimos make igloos out of snow because snow traps air, which is an insulator, keeping the heat inside and making the igloo warm.
- Mud houses with thatched roofs stay cool in summer and warm in winter because mud and thatch are bad conductors of heat.
- Ice boxes have double walls with glass wool between them, which is a bad conductor, so the ice inside does not melt quickly.
- Handles of kettles, electric irons, and cooking pans are made of wood or plastic because they are bad conductors, so we can hold them without burning our hands.
- Ice slabs are covered with sawdust or gunny bags because these materials are bad conductors and stop heat from reaching the ice.
- In cold countries, double glass window panes are used with air trapped between them because air is an insulator, keeping the room warm.
Convection
- Convection is the process where particles in a liquid or gas move and carry heat energy from a hot area to a cold area.
- In convection, the particles actually move to the source of heat, take in heat, and then move away to spread the heat.
- Convection happens in liquids and gases, but not in solids because solids do not allow particles to move freely.
- Mercury is an exception because, even though it is a liquid, it conducts heat well and does not use convection much.
Application of Convection Currents in Gases
Ventilation
- Ventilation is the process of bringing fresh air into a room and removing warm air to keep the room comfortable.
- When people breathe, they release moisture and carbon dioxide into the air, making it warm and light.
- This warm air rises and goes out through ventilators (openings near the ceiling).
- Cooler, fresh air from outside comes in through doors and windows to replace the warm air.
- This continuous movement of air, called a convection current, keeps the room fresh and cool.
Radiation
- Radiation is the transfer of heat energy directly from a hot object to a cold object without needing any medium (like air, water, or solids) in between.
- Heat energy travels as radiant heat at a speed of 3 x 108 m/s (very fast).
- Radiant heat does not need conduction or convection to travel because it can move through empty space (a vacuum).
- For example, the heat from the sun reaches Earth through space, even though space has no air or matter.
Radiant Heat or Thermal Radiation
- When a hot object gives off heat to a cold object through radiation, it is called radiant heat or thermal radiation.
- Radiant heat travels in space at a speed of 3 x 108 m/s.
- When radiant heat hits a solid object, some of it is absorbed, which raises the object’s temperature.
- The part of the heat that is absorbed depends on the material of the object.
- The part that is not absorbed is reflected back.
- Darker or black objects absorb more radiant heat, so their temperature rises more.
- White or shiny objects reflect more heat and absorb less, so their temperature rises less.
- Black objects are good absorbers of radiant heat, while white or shiny objects are poor absorbers (or good reflectors).
- Black objects are also good radiators of heat, meaning they give off heat easily.
- White or shiny objects are poor radiators of heat, meaning they do not give off heat easily.
Everyday Applications of Radiant Heat
- We wear white or light-colored clothes in summer because they are poor absorbers of radiant heat, keeping us cool.
- Car engines are painted black so they can radiate heat quickly and keep the engine cool.
- We wear dark or black clothes in winter because black is a good absorber of heat, keeping us warm.
- Water tanks are painted white because white is a poor absorber of radiant heat, so the water stays cool in summer.
- Cooking pots are blackened at the bottom but shiny on the sides; the black bottom absorbs heat quickly, while the shiny sides trap the heat inside.
- Firemen wear shiny helmets because shiny surfaces are poor absorbers and good reflectors of heat, protecting them from heat.
Thermos Flask or Vacuum Flask
- A thermos flask is used to keep a hot liquid hot or a cold liquid cold for a long time.
- It is a double-walled glass container with a vacuum (empty space) between the two walls.
- The outer surface of the inner wall and the inner surface of the outer wall are silvered (shiny).
- The flask is placed in a metal case, sealed with a cork, and closed with a plastic cup and rubber ring.
- The vacuum between the walls stops heat from moving by conduction or convection because there is no air to carry the heat.
- The silvered surfaces stop heat from moving by radiation because they are poor radiators and good reflectors.
- The glass walls, cork, and rubber are bad conductors of heat, so they do not let heat pass through easily.
- The cork in the neck and the plastic cup also stop heat from escaping by conduction or convection.
- This design ensures that a hot liquid stays hot and a cold liquid stays cold for a long time.
Points To Remember
|
26 videos|121 docs|37 tests
|
FAQs on Heat Chapter Notes - Science for Grade 4
| 1. What is heat energy and what are its general effects? |  |
| 2. How is temperature measured and what are the common instruments used? |  |
| 3. What are the different temperature scales and how can they be converted? |  |
| 4. What are some numerical problems related to temperature and its measurement? |  |
| 5. What interesting facts can be found about heat energy and temperature? |  |