Water Chapter Notes | Chemistry for SSS 1 PDF Download
Introduction
Water, the elixir of life, covers more than 70% of Earth's surface and is a vital component of all living organisms. This fascinating chapter dives into the unique properties of water, its role as a universal solvent, and its interactions with various substances. From the water cycle to the formation of stalactites in caves, explore how water shapes our world. Learn about solutions, hydrated and anhydrous substances, and the differences between soft and hard water. Get ready to uncover the science behind this essential resource and its incredible impact on life and the environment!
- Only 2.5% is fresh water, including frozen water in polar ice caps and glaciers.
- Present in all living organisms, making up nearly 70% of human body weight.
- Occurs in free and combined states.
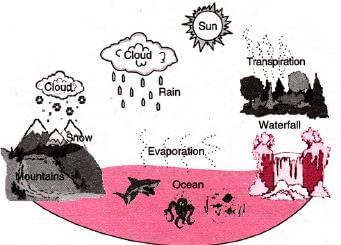
- Water cycle illustrates movement and transformation of water in nature.
Water
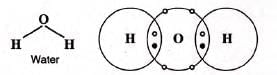
- Chemical formula: H2O
- Chemical name: Dihydrogen oxide
- Molecular mass: 2 × 1 + 16 × 1 = 18 amu
- Exists in three physical states: solid (ice), liquid (water), and gas (water vapor).
Physical Properties of Water
Nature:
- Pure water is clear, transparent, colorless, odorless, and tasteless.
- Taste comes from dissolved gases and solids (impurities).
Boiling point:
- Boils at 100°C under normal pressure (760 mm Hg).
- Boiling point increases with higher pressure, as in pressure cookers.
- In hills, boils below 100°C (e.g., 70°C on Mount Everest) due to lower pressure.
- Dissolved impurities also raise the boiling point.
Freezing point:
- Freezes at 0°C under normal pressure (1 atmosphere).
- Freezing point decreases with increased pressure or dissolved impurities.
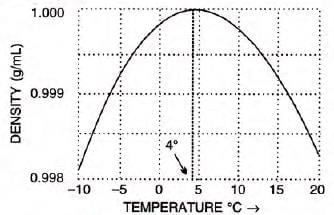
Density: Maximum density of 1 g/cm3 or 1000 kg/m3 at 4°C, with minimum volume.
Water expands on freezing:
- 92 volumes of water become 100 volumes of ice.
- Relative density of ice is 0.92, allowing it to float.
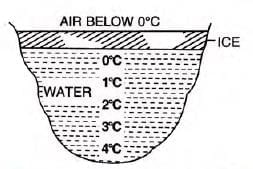
Anomalous expansion of water:
- Contracts when cooled until 4°C, then expands until it freezes at 0°C.
- This property allows marine life to survive in cold regions, as water remains liquid below the ice layer.
Latent heat of fusion of ice: Heat required to change ice to water at 0°C without temperature change.
Specific value:
- 336 J/g or 80 cal/g.
- Same heat is released when 1 g of water freezes to ice at 0°C.
- High latent heat prevents sudden freezing of lakes and rivers.
Latent heat of vaporization of water: Energy needed to convert water to steam at 100°C without temperature change.
Specific value:
- 2268 J/g or 540 cal/g.
- Same heat is released when 1 g of steam condenses to water at 100°C.
- High latent heat causes severe burns from steam compared to boiling water.
Specific heat capacity:
- 1 g of water absorbs 4.2 J (1 calorie) of heat to rise 1°C.
- High specific heat makes water a great coolant for car radiators, desert coolers, etc.
- Moderates climate of nearby land, making winters warmer and summers cooler.
- Drives land and sea breezes due to its heat-retaining property.
Water as Universal Solvent
- Water dissolves many substances, forming aqueous solutions.
- High dielectric constant reduces attraction between positive and negative ions, dissolving inorganic compounds.
- Dissolves solids, gases, and other liquids, earning the title "universal solvent."
- Even "insoluble" substances dissolve in traces; glass etches when distilled water is stored long-term.
- Experiment: To show tap water contains dissolved solids.
- Procedure: Place tap water on a watch glass, heat over a beaker of boiling water until it evaporates.
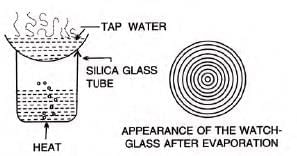
- Observation: Concentric rings of solid matter (dissolved solids) remain after evaporation.
Importance of dissolved salts in water
- Essential for plant growth and development.
- Add taste to water.
- Supply vital minerals for the human body.
- Note: Rainwater and distilled water lack dissolved solids, so no rings form.
Air dissolved in water
Natural water contains dissolved air, with oxygen more soluble than nitrogen.- Composition: 33% oxygen, 66% nitrogen, 1% carbon dioxide (unlike 21% oxygen in air).
- Experiment: To show tap water contains dissolved gases.
- Procedure: Heat tap water in a flask, collect escaping gas in a graduated tube via downward displacement of water.
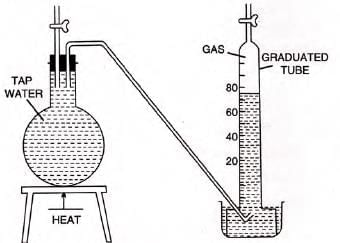
- Observation: Gas bubbles escape, water vapor condenses, remaining gas is dissolved air.
- Solubility example: 0.02 volumes of H2 or 506 volumes of HCl per unit volume of water at 0°C.
- Note: Boiling expels dissolved gases, making boiled or distilled water tasteless.
Importance of air dissolved in water
- Marine life (e.g., fish) uses dissolved oxygen for respiration; 1 dm3 water holds 40 cm3 oxygen.
- Aquatic plants use dissolved CO2 for photosynthesis: 6 CO2 + 12 H2O → C6H12O6 + 6 O2 + 6 H2O.
- CO2 reacts with limestone: CaCO3 + CO2 + H2O → Ca(HCO3)2.
- Marine organisms (e.g., snails, oysters) use calcium bicarbonate to build shells.
Solutions as Mixtures of Solids in Water
- A solution is a uniform mixture of two or more components, adjustable by varying amounts.
- Formula: Solution = Solute + Solvent.
- Solvent: Medium that dissolves the solute to form a solution.
- Solute: Substance that dissolves in the solvent.
- Binary solution: Made of two components.
- Ternary and quaternary solutions have three or four components, respectively.
- Example: In saline water, salt is the solute, water is the solvent.
- If components differ in state, the solvent matches the solution’s state (e.g., water in sugar solution).
- If states are the same, the component in excess is the solvent.
Types of solutions:
- Liquid + liquid: e.g., water and alcohol.
- Solid + liquid: e.g., sugar solution.
- Liquid + gas: e.g., soda water.
- Solid + solid: e.g., alloys.
- Solid solutions: Homogeneous mix of solids, like brass (70% copper, 30% zinc).
Characteristics of a true solution:
- Uniform mixture.
- Solute particles are tiny, about 10-10 m.
- Clear and transparent.
- Does not scatter light.
- Cannot be separated by filtration.
- Solute particles do not settle.
- Note: Water is a common solvent, forming aqueous solutions.
- Non-aqueous solvents: Alcohol, petrol, ether, benzene, carbon disulfide, liquid ammonia.
- Tincture: Solution with alcohol as solvent, e.g., tincture of iodine (iodine in alcohol).
- Dilute solution: Small amount of solute relative to solvent.
- Concentrated solution: Large amount of solute for a given solvent mass.
Saturated Solution
A solution that cannot dissolve more solute at a given temperature.- Preparation: Add 1 g of nitre to 100 g water, stir to dissolve, continue until no more dissolves.
- Result: Solution is saturated at that temperature.
- Cooling a saturated solution: Solubility drops, excess solute forms crystals.
- Unsaturated solution: Can dissolve more solute at that temperature.
- Supersaturated solution: Holds more solute than theoretically possible at room temperature.
- Preparation of supersaturated solution: Heat a saturated solution, add more solute, cool back to room temperature.
- Note: Cooling without crystals requires no impurities and no disturbance.
Concentration of a Solution
Measures amount of solute in a given quantity of solution.
Mass percent: Mass of solute in grams per 100 g of solution.
- Formula:
Concentration

- Example: 10 g sodium chloride in 90 g water
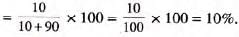
Volume percent: Volume of solute in mL per 100 mL of solution.
- Formula:
Volume percent
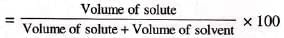
- Example: 30 mL alcohol in 70 mL water
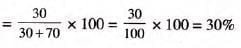
Solved Example:
- Example 1:2-5 litres of alcohol is present in 10 litres of aqueous solution of alcohol. Calculate volume percent.
Volume of solute = 2-5 litres
Volume of solution = 10 0 litres
- Solution:
Volume percent

- Example 2: 50 gram of sugar is dissolved in 245 kg of water. Calculate the concentration of solution.
- Solution:
Mass of solute = 50 gram,
mass of solvent = 2450 gram,
mass of solution = mass of solute + mass of solvent = 2500 gram
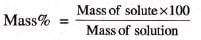
Mass % = (50 / 2500) × 100 = 2%.
Solubility
Different substances dissolve to varying extents in the same solvent.- Example: At 40°C, 36.5 g sodium chloride and 65 g potassium nitrate dissolve in 100 g water.
- Solubility: Maximum grams of solute to saturate 100 g of solvent at a given temperature.
- Insoluble: Negligible solubility, e.g., silver chloride (0.000015 g).
- Sparingly soluble: Low but noticeable solubility, e.g., calcium hydroxide (0.17 g).
- Soluble: High solubility, e.g., sodium chloride.
Determination of the solubility of a solute at a particular temperature
- Note temperature of saturated solution.
- Weigh empty evaporating dish = M.
- Add saturated solution, weigh again = M1.
- Heat to dryness, weigh = M2.
- Mass of solution = M1 - M.
- Mass of solute = M2 - M.
- Mass of solvent = (M1 - M) - (M2- M).
At the noted temperature :
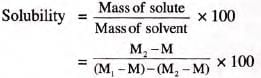
Factors affecting solubility
- Size of solute particles: Smaller particles have greater surface area, increasing solubility.
- Stirring: Brings more solvent in contact with solute, speeding up dissolution.
- Temperature: Solubility of gases decreases with rising temperature.
- Solubility of most solids increases with temperature, e.g., potassium nitrate: 31.6 g at 20°C, 63.9 g at 60°C.
Solubility curves
- Line graph showing solubility changes with temperature.
- Solubility on y-axis, temperature on x-axis.
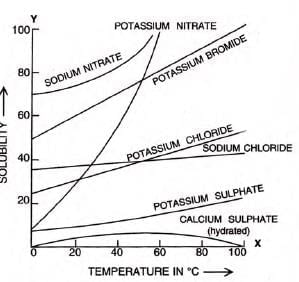
- Calcium sulphate (CaSO4·2H2O) solubility decreases after a certain temperature.
- Sodium nitrate, potassium nitrate, and potassium bromide show significant solubility increase with temperature.
- Sodium chloride solubility increases slightly with temperature.
- In endothermic processes, solubility rises with temperature (e.g., KNO3).
- In exothermic processes, solubility increases with lower temperature (e.g., calcium hydroxide).
Anomalous solubility:
- Some salts (e.g., Na2SO4·10H2O) increase then decrease with temperature.
- Solubility of Na2SO4·10H2O peaks at 32.8°C, then falls as it becomes anhydrous.
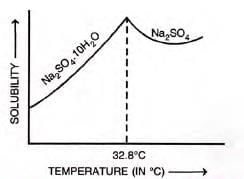
Applications of solubility curves
- Show how solubility varies with temperature.
- Determine solubility at a specific temperature.
- Compare solubilities of different solutes on the same chart.
- Predict effects of cooling hot solutions: some salts precipitate, others become unsaturated.
Some solved examples:
- Example 1: 12 g of a saturated solution of potassium chloride at 20 °C, when evaporated to dryness, leaves a solid residue of 3 g. Calculate the solubility of potassium chloride.
- Solution: Weight o f water in solution = 1 2 g - 3 g = 9 g
9 g of H2O dissolves 3 g of solid ∴ 100 g of water will dissolve 3/9 x 100 = 33.3 g
- Example 2: Weight of sodium nitrate for 60 g crystals, solubility 140 g at 70°C, 100 g at 25°C.
- Solution: Crystals from cooling = 140 - 100 = 40 g; For 60 g, need (140 / 40) × 60 = 210 g.
Effect of pressure and temperature on solubility of gases in water (liquids)
- Pressure: Higher pressure increases gas solubility in water.
- Henry’s Law: Mass of gas dissolved in a liquid is proportional to pressure on the liquid’s surface.
- Example: More CO2 dissolves in soda water under high pressure; bubbles escape when pressure drops.
- Temperature: Higher temperature reduces gas solubility, making boiled water tasteless.
Crystals and Crystallization
- Crystal: Homogeneous solid with a definite geometric shape and smooth plane surfaces.
- Crystallization: Process of forming crystals by cooling a hot saturated solution.
- Crystal shapes: Cubic (NaCl), rhombohedric (CuSO), octahedral (FeSO4), prismatic (KNO3).
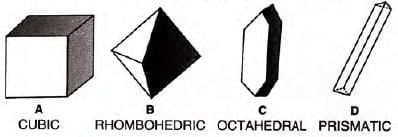
- Natural crystals: Common salt, potassium nitrate, ruby, sapphire, diamond.
- Colors may come from water or impurities, e.g., red ruby from mercury oxide.
Methods to obtain crystals:
- Cooling a hot saturated solution.
- Slowly evaporating a saturated solution.
- Cooling a fused mass.
- Sublimation.
Experiment: To prepare large copper sulphate crystals.
- Procedure: Make saturated CuSO4 solution at 80°C, filter, cool, and suspend a small crystal in it.
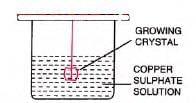
- Observation: Crystal grows larger over days; seeding induces crystallization.
- Caution: Avoid dust to prevent unwanted crystal formation.
Hydrated and Anhydrous Substances
- Hydrated substances contain water molecules, e.g., Na2CO3·10H2O, CuSO4·5H2O.
- Anhydrous substances lack water, e.g., NaCl, KNO3.
Hydrated substances
Crystals with water of crystallization, giving shape and sometimes color (e.g., blue CuSO4).
Water of crystallization: Fixed water in hydrated crystals, loosely bonded.
Examples: Washing soda (Na2CO3·10H2O), Epsom salt (MgSO4·7H2O), blue vitriol (CuSO4·5H2O).
Experiment: To show hydrated copper sulphate contains water.
- Procedure: Heat CuSO4·5H2O in a tilted test tube.
- Observation: Colorless liquid condenses, residue becomes white, amorphous anhydrous CuSO4.
- Reaction:
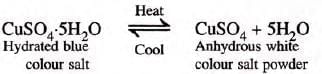
Determination of water of crystallization:
- Weigh crystals = a g.
- Heat above 100°C until weight is constant = b g.
- Water weight = (a - b) g.
- % of water = [(a - b) / a] × 100.
Anhydrous substances
- Substances without water, often crystalline salts after water removal.
- Examples: Table salt (NaCl), gaseous HCl.
Methods to obtain anhydrous salts:
- Direct heating.
- Heating in dry, hot air.
- Heating under vacuum.
- Using dehydrating agents like warm concentrated H2SO4.
- Crystalline substances without water: NaCl, KNO3, sugar (C12H22O11), KMnO4, NH4Cl.
Experiment: Action of heat on anhydrous NaCl and KNO3.
- Procedure: Heat NaCl and KNO3 in separate test tubes.
- Observation: NaCl crackles (decrepitation) as crystals break; KNO3 melts, releases O2, leaves light yellow KNO2.
- Reaction:
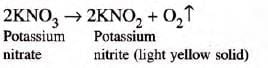
- Conclusion: Both the substances do not contain water of crystallization.
Properties
Efflorescence
- Phenomenon where hydrated crystals lose water to dry air, crumbling to powder.
- Occurs when vapor pressure in crystals exceeds atmospheric vapor pressure.
- More pronounced in hot, dry conditions.
Examples:
- Washing soda: [h y d ra te d so d iu m c a rb o n a te ], when exposed to dry air, becomes a monohydrate.
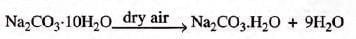
- Glauber’s salt: [hydrated sodium sulphate, Na2SO4.10H2O] becomes a powdery anhydrous sodium sulphate when exposed to air.
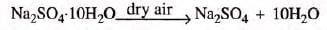
- Epsom salt: [magnesium sulphate heptahydrate], when exposed to dry air, becomes a monohydrate.
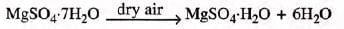
Deliquescence
- Water-soluble substances absorb moisture from air, become wet, and dissolve into a solution.
- Occurs when vapor pressure in crystals is lower than atmospheric vapor pressure.
- Reduced in dry conditions.
Deliquescent substances:
- Examples: Caustic soda (NaOH), caustic potash (KOH), MgCl2, ZnCl2, CaCl2, FeCl3.
- Note: Table salt turns moist due to deliquescent impurities (MgCl2, CaCl2).
- Purify by passing dry HCl gas through saturated solution, filtering pure NaCl.
Hygroscopic Substances
- Substances that absorb moisture from air without dissolving.
- Examples: Concentrated H2SO4, phosphorus pentoxide (P2O5), quicklime (CaO).
Drying and Dehydrating Agents
- Drying agents: Absorb moisture without chemical reaction.
- Examples: Anhydrous CaCl2, ZnCl2, P2O5, MgSO4, dry Na2SO4.
- Also called desiccants.
- Dehydrating agents: Remove chemically combined water from compounds.
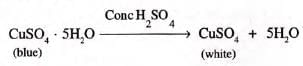
- Example:
Concentrated H2SO4dehydrates
Drying gases:
- Pass through concentrated H2SO4 for acidic gases like HCl.
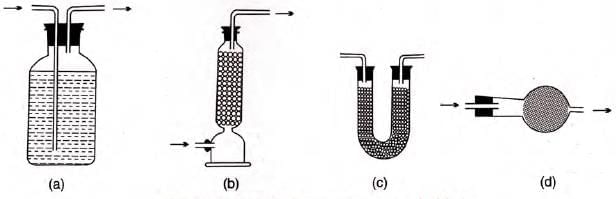
- Pass through drying tower or U-tube with anhydrous Na2SO4.
- Use drying bulb with anhydrous CaCl2.
- Quicklime (CaO) dries basic gases like NH3.
- Drying liquids: Keep overnight with anhydrous Na2SO4, MgSO4, or CaCl2, then filter.
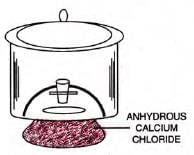
- Drying solids: Spread on watch glass or dish, keep in desiccator with anhydrous CaCl2.
- Desiccator: Air-tight glass vessel for drying solids.
Drying and Dehydrating Agents
Drying agent properties:
- Remove moisture from substances.
- Dry gases like chlorine, sulfur dioxide, hydrogen chloride.
- Used in desiccators to keep substances dry.
- Physical change.
- Examples: P2O5, fused CaCl2, CaO, concentrated H2SO4.
Dehydrating agent properties:
- Remove water elements (H:O in 2:1 ratio) from compounds.
- Prepare substances like carbon monoxide, sugar charcoal.
- Example:
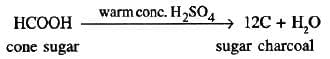
- Chemical change.
- Example: Concentrated H2SO4.
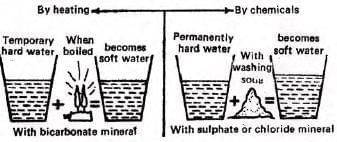
Soft and Hard Water
- Soft water: Readily forms lather with soap; includes pure water or water with sodium salts.
- Examples: Distilled water, rainwater.
- Hard water: Does not easily form lather with soap.
Causes of hardness
- Contains dissolved minerals like bicarbonates, sulfates, or chlorides of calcium and magnesium.
- Water flowing over limestone (CaCO3) or dolomite (MgCO3) forms soluble bicarbonates.
- Reaction: CaCO3 + CO2 + H2O → Ca(HCO3)2.
- Stalactites (from cave roof) and stalagmites (from floor) form from calcium carbonate deposits.
- Gypsum (CaSO4·2H2O) dissolves slightly, making water hard.
Types of Hardness Temporary and Permanent Hardness
- Temporary hard water: Contains only calcium and magnesium bicarbonates.
- Hardness removed by boiling.
- Reactions:
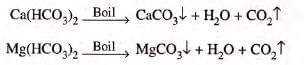
- Permanent hard water: Contains sulfates and chlorides of magnesium and calcium.
- Note : The removing of temporary hardness of water by boiling is, however, not a practical method; it is costly and slow.
Advantages of hard water
Hard water containing some dissolved salts has the following advantages.
- Soft water tastes flat; dissolved salts in hard water add taste, useful for beverages and wines.
- Calcium and magnesium salts support bone and teeth growth.
- Hard water prevents lead poisoning in pipes by forming insoluble lead sulfate layer.
Disadvantages of hard water
- Unfit for steam production in boilers.
- Dissolved solids deposit as scale in boiler tubes, narrowing them and reducing steam output.
- Scale, a poor heat conductor, causes heat loss; high pressure may burst boiler.
- Boiler scale removed with compounds or softened water, both costly.
- Furring in tea kettles from carbonate deposits.
- Unfit for washing: Soap (C17H35COONa) reacts with Ca and Mg ions, forming soap curd.
- Reaction: 2 NaSt + Ca(HCO3)2 → CaSt2↓ + 2 NaHCO3.
- Curd prevents lather, sticks to fabrics, may cause rusty spots if iron salts present.
- Synthetic detergents (e.g., SURF, DET) solve this, being soluble and unaffected by hardness.
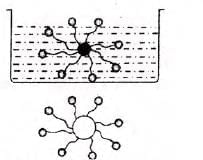
Cleansing action of soaps and detergents:
- Molecules form micelles, with tails inward and heads outward.
- Tails attach to oily dirt, water stirs to lift dirt, breaking it into fragments.
- Detergent molecules surround oil globules, negative heads prevent re-aggregation.
- Oily dirt is removed from surfaces.
Removal of hardness
Removal of temporary hardness:
- Boiling: Expels CO2, converts bicarbonates to insoluble carbonates.
- Reaction: Ca(HCO3)2 → CaCO3↓ + H2O + CO2↑.
- Filter or decant to remove precipitates.
- Clark’s Process: Add slaked lime to precipitate carbonates.
- Reactions:
Ca(HCO3)2 + Ca(OH)2 → 2 CaCO3↓ + 2 H2O.
Mg(HCO3)2 + Ca(OH)2 → MgCO3↓ + CaCO3↓ + 2 H2O.
- Mix lime with water, add to hard water, filter out precipitates.
- Add washing soda: Converts bicarbonates to insoluble carbonates.
- Reactions:
Ca(HCO3)2 + Na2CO3 → CaCO3↓ + 2 NaHCO3.
Mg(HCO3)2 + Na2CO3 → MgCO3↓ + 2 NaHCO3.
Removal of permanent hardness:
Treat with soda ash to form insoluble carbonates.
- Reactions:
CaSO4 + Na2CO3 → CaCO3↓ + Na2SO4.
CaCl2 + Na2CO3 → CaCO3↓ + 2 NaCl. - For MgSO4, use lime and soda ash: MgSO4 + Na2CO3 + Ca(OH)2 → Mg(OH)2↓ + CaCO3↓ + Na2SO4.
- Permutit process: Uses artificial zeolite (Na2Al2Si2O8·XH2O, or Na2P).
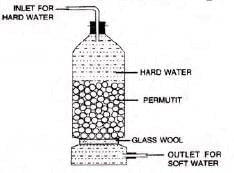
- Hard water passes through permutit, exchanging Ca and Mg ions for Na ions.
- Regenerate permutit with brine: CaP + 2 NaCl → Na2P + CaCl2.
- Ion exchange resins: Remove all ions for scientific use.
- Cation resins replace Na, Ca, Mg with H+; anion resins replace HCO3-, SO42- with OH-.
- Result: Completely deionized water.
|
19 videos|100 docs|23 tests
|
FAQs on Water Chapter Notes - Chemistry for SSS 1
| 1. What are the physical properties of water and why are they important? |  |
| 2. What makes water a universal solvent? |  |
| 3. What is a saturated solution and how can it be recognized? |  |
| 4. What is the difference between hydrated and anhydrous substances? |  |
| 5. What are drying and dehydrating agents, and how do they function? |  |





















