Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Mind Map: Mole Concept and Stoichiometry
Mind Map: Mole Concept and Stoichiometry | Chemistry Class 10 ICSE PDF Download
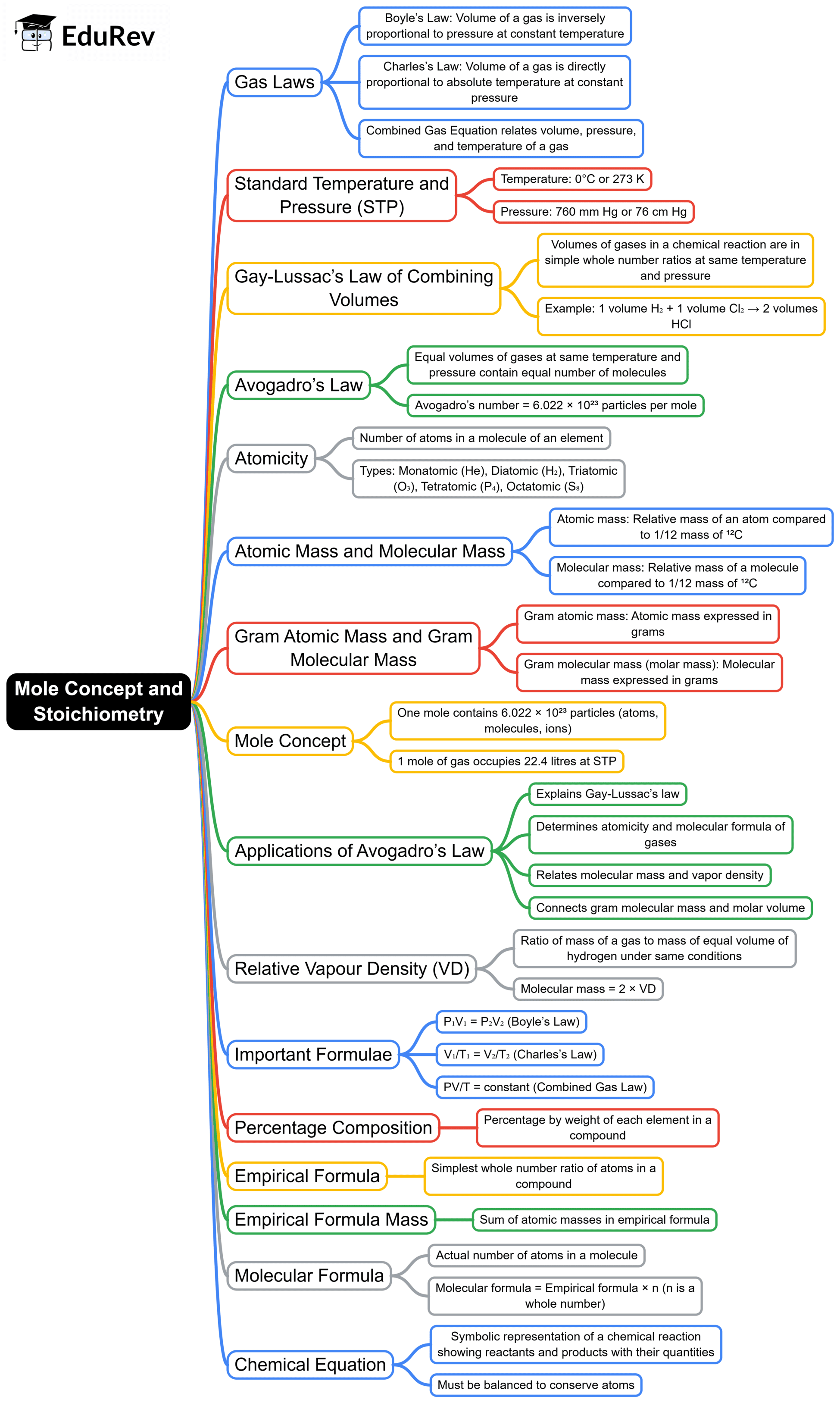
The document Mind Map: Mole Concept and Stoichiometry | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
39 videos|85 docs|14 tests
|
FAQs on Mind Map: Mole Concept and Stoichiometry - Chemistry Class 10 ICSE
| 1. What is the mole concept and why is it important in chemistry? |  |
Ans. The mole concept is a fundamental principle in chemistry that allows chemists to count particles, such as atoms, molecules, or ions, in a given sample. One mole of any substance contains approximately 6.022 × 10²³ particles, known as Avogadro's number. This concept is crucial because it provides a bridge between the atomic scale and the macroscopic scale, enabling chemists to perform calculations involving mass, volume, and number of particles in chemical reactions.
| 2. How do you calculate the number of moles from mass and molar mass? |  |
Ans. To calculate the number of moles from mass and molar mass, you can use the formula: Number of moles = Mass (in grams) / Molar mass (in grams per mole). For example, if you have 18 grams of water (H₂O) and the molar mass of water is 18 g/mol, then the number of moles of water is 18 g / 18 g/mol = 1 mole.
| 3. What is stoichiometry and how is it related to the mole concept? |  |
Ans. Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It is closely related to the mole concept because stoichiometric calculations often require the use of moles to determine how much of each substance is involved in the reaction, allowing for accurate predictions of product yields and reactant consumption.
| 4. How can you determine the empirical formula from percent composition? |  |
Ans. To determine the empirical formula from percent composition, first convert the percentage of each element to grams (assuming a 100 g sample). Then, convert the mass of each element to moles by dividing by the atomic mass. Next, find the simplest whole number ratio of moles of each element. This ratio gives you the empirical formula. For example, if you have 40% carbon and 60% oxygen, you would convert these to moles and find the simplest ratio to derive the empirical formula.
| 5. What is the significance of balancing chemical equations in stoichiometry? |  |
Ans. Balancing chemical equations is significant in stoichiometry because it ensures that the law of conservation of mass is upheld, meaning that the mass of reactants equals the mass of products. A balanced equation provides the correct mole ratios of reactants and products, which is essential for calculating the amounts needed in a chemical reaction and predicting the outcomes of the reaction accurately.
Related Searches
















