Short & Long Question Answers:: Chemical Bonding and Molecular Structure | Chemistry Class 11 - NEET PDF Download
Short Answer Type Questions
Q1: Explain the non linear shape of H2S and non planar shape of PCl3 using valence shell electron pair repulsion theory.
Ans: The main atom in H2S is S, which has two lone pairs. These lone pairs cause repulsion and displace the H-S bond, resulting in a non-linear shape.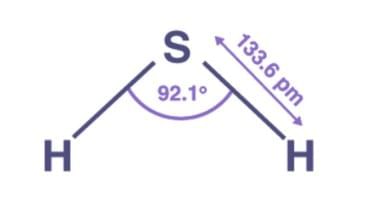
PCl3 has a trigonal planar structure. P has three single bonds and one lone pair (pair of unshared electrons). Each chlorine atom has a single 3p orbital that is completely occupied. The overlap of a phosphorus sp3 hybrid orbital with a singly occupied chlorine 3p orbital results in the formation of P–Cl bonds. Three lone pairs are held by each Cl atom.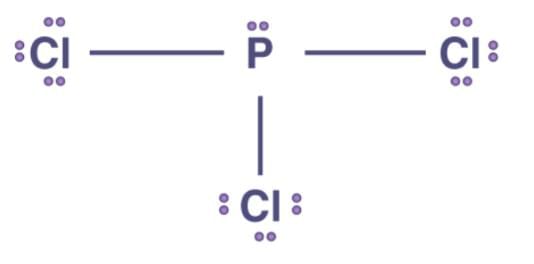
Q2: Explain the shape of BrF5.
Ans: In BrF5 the central atom Br is surrounded by five bonded pairs and one lone pair. This forms the shape of square pyramidal.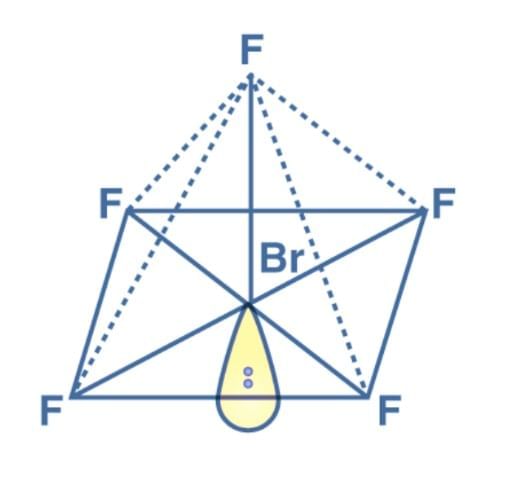
Q3: In both water and dimethyl ether (CH3–O–CH3), oxygen atom is central atom, and has the same hybridization, yet they have different bond angles. Which one has a greater bond angle? Give reason.
Ans: The bond angle of dimethyl ether will be greater. More repulsion will exist between bond pairs of CH3 groups attached in ether than between bond pairs of hydrogen atoms attached to oxygen in the water.
The carbon of CH3 in ether is attached to three hydrogen atoms via bonds, and the electron pair of these bonds contribute to the electronic charge density on the carbon atom. As a result, the repulsion between two CH3 groups will be greater than that between two hydrogen atoms.
Q4: Explain why PCl5 is trigonal bipyramidal whereas IF5 is square pyramidal.
Ans: P is surrounded by 5 bond pairs and no lone pairs in PCl5, whereas iodine atom is surrounded by 5 bond pairs and one lone pair in IF5, so the shape of PCl5 is trigonal bipyramidal and IF5 is square pyramidal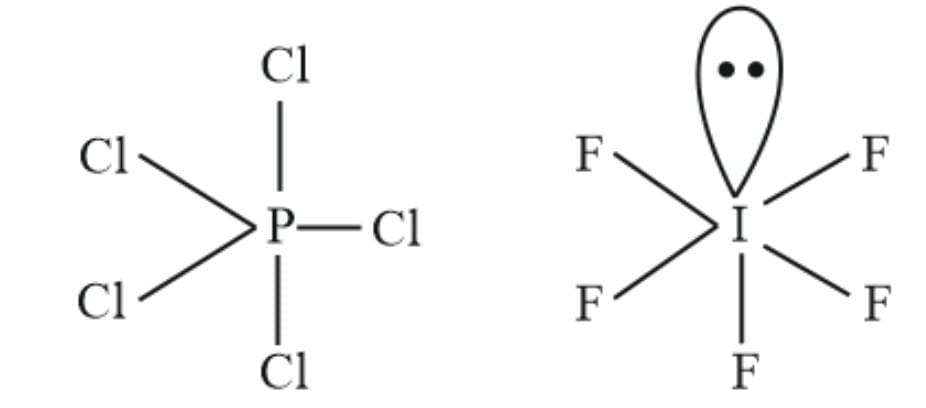
Q5: Write Lewis structure of the following compounds and show a formal charge on each atom. HNO2, NO2, H2SO4
Ans: Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
The Lewis structure of HNO3 is-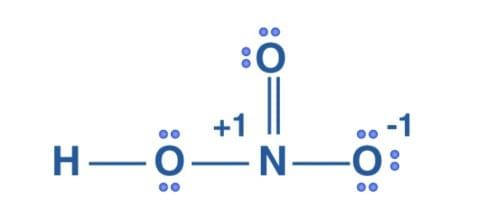
Formal charge on O that has double bond = 6 – 4 –½ (4) = 0
Formal charge on O that is attached to H atom = 6 – 4 –½ (4) = 0
Formal charge on O that has single bond = 6 – 6 –½ (2) = – 1
Formal charge on H = 1 – 0 – ½ (2) = 0
Formal charge on N = 5 – 0 – ½ (8) = +1
The Lewis structure of NO2 is-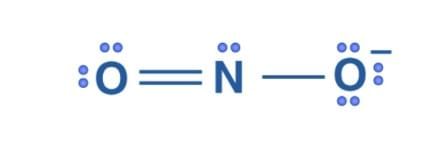
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
Formal charge on O that has double bond = 6 – 4 –½ (4) = 0
Formal charge on O that has single bond = 6 – 6 –½ (2) = – 1
Formal charge on N = 5 – 2 – ½ (6) = 0
The Lewis structure of H2SO4 is-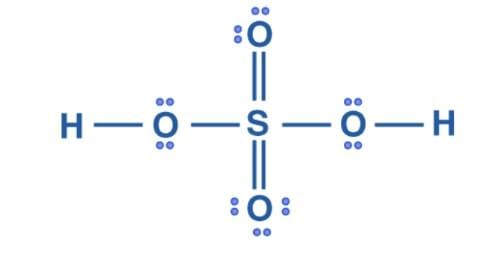 Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
Formal charge on O that has double bond = 6 – 4 –½ (4) = 0
Formal charge on O that has single bond = 6 – 4 –½ (4) = 0
Formal charge on H = 1 – 0 – ½ (2) = 0
Formal charge on S = 6 – 0 – ½ (12) = 0
Q6: What is the effect of the following processes on the bond order in N2 and O2?
(i) N2 → N2+ + e–
(ii) O2 → O2+ + e–
Ans:
(i) N2 → N2+ + e–
The bond order in N2 is 3 while that in N2+ is 2.5. This indicates that the bond order decreases.
(ii) O2 → O2+ + e–
The bond order in O2 is 2 while that in O2+ is 2.5. This indicates that the bond order increases.
Q7: Give reasons for the following:
(i) Covalent bonds are directional bonds while ionic bonds are nondirectional.
(ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(iii) Ethyne molecule is linear.
Ans: (i) A covalent bond is formed by the overlapping of half-filled atomic orbitals with definite directions, i.e., shared electron pair/pairs are localised between two atoms. As a result, a covalent bond is also known as a directional bond. Since each ion in an ionic compound has an influence in all directions, it is surrounded by a number of oppositely charged ions with no definite direction and, therefore, is non-directional.
(ii) The central oxygen atom in water is sp3 hybridised, whereas the central carbon atom in CO2 is sp-hybridised. The net dipole moment of CO2 is zero, whereas H2O has a significant value. This demonstrates that CO2 has a linear structure, whereas water has a bent structure.
(iii) Each carbon atom in ethyne is sp-hybridized, resulting in a linear structure.
Q8: What is an ionic bond? With two suitable examples explain the difference between an ionic and a covalent bond?
Ans: Ionic bonds are chemical bonds formed between two atoms as a result of the transfer of one or more electrons from one atom to the other. Such a bond is only possible between atoms of different characteristics, with one atom having a tendency to lose electrons and the other atom having a tendency to accept electrons.
Distinctive features:
(i) Ionic bonds can form between dissimilar atoms, such as electropositive and electronegative atoms, whereas covalent bonds can form between similar and dissimilar atoms.
(ii) An ionic bond is neither rigid nor directional. It does not exhibit isomerism, whereas a covalent bond is rigid and directional, causing space isomerism.
Q9: Arrange the following bonds in order of increasing ionic character giving reason.
N – H, F – H, C – H and 0 – H.
Ans: When there is a sufficient difference in the electronegativity of the two atoms, the ionic character is observed in a covalent bond.
Ionic character ∝ Electronegativity difference.
The following is an order of increasing ionic character.
C – H < N – H < O – H < F – H
Q10: Explain why CO22– ion cannot be represented by a single Lewis structure. How can it be best represented?
Ans: In the carbonate ion CO32–. The lengths of the three C to O bonds are all the same. A single Lewis structure cannot demonstrate this. The ion is a composite of three different structures.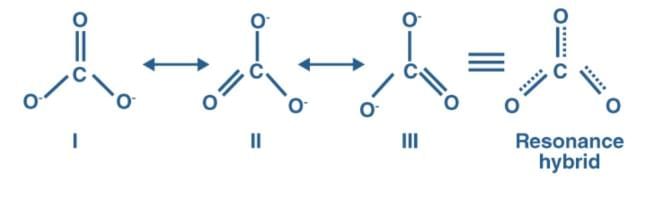
Long Answer Type Questions
Q1: i) Discuss the significance/ applications of dipole moment.
(ii) Represent diagrammatically the bond moments and the resultant dipole moment in CO2, NF3 and CHCI3.
Ans: i) Dipole moment (μ) = charge (Q) ✕ separation distance (r). Debye units are commonly used to express dipole moment (D).
(a) It aids in the prediction of the polar and non-polar nature of compounds. Non-polar molecules have no dipole moment, whereas polar molecules have a dipole moment.
(b) It is possible to predict the nature of a chemical bond formed by knowing the electronegativities of the atoms involved in a molecule. The bond will be highly polar if the difference in electronegativities between two atoms is large. An ionic bond is formed when an electron is completely transferred from one atom to another.
The following formula can be used to calculate the percentage of ionic characters:
(c) It is beneficial to understand the symmetry of the molecule. Despite having two or more polar bonds, symmetrical molecules have zero dipole moment.
In the case of BeF2, for example, the dipole moment is zero. This is due to the fact that the two equal bond dipoles point in opposite directions and cancel each other out.
(d) Differentiate between cis- and trans-isomers. Dipole moment measurements aid in the differentiation of cis- and trans-isomers because the ds-isomer has a higher dipole moment than the trans isomer.
(e) Distinguish between ortho, meta and para isomers. Dipole moment measurements aid in the differentiation of o-, m-, and p-isomers because the dipole moment of the p-isomer is zero and that of the o-isomers is greater than that of the m-isomer.
(ii) The bond moments and the resultant dipole moment in CO2, NF3 and CHCI3.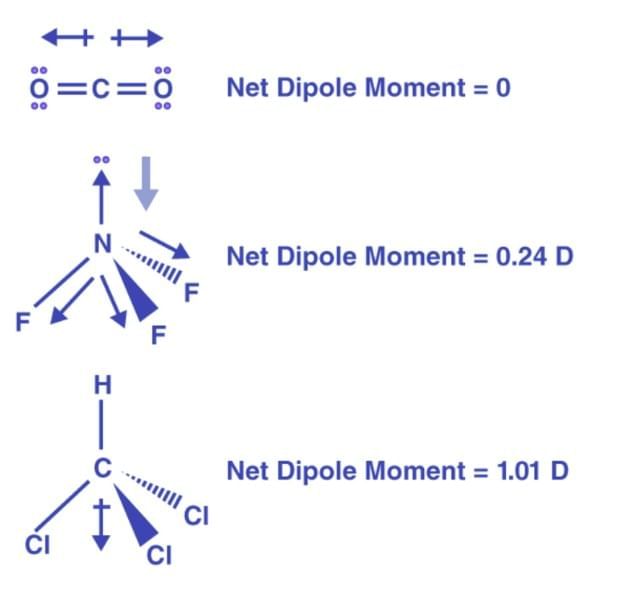
Q2: Describe hybridization in the case of PCl5 and SF6. The axial bonds are longer as compared to equatorial bonds in PCl5 whereas in SF6, both axial bonds and equatorial bonds have the same bond length. Explain.
Ans: The ground state electronic configuration of P (15)- 1s2 2s2 3s2 3p3 3d0
The excited state outer electronic configuration- 1s2 2s2 3s1 3p3 3d1
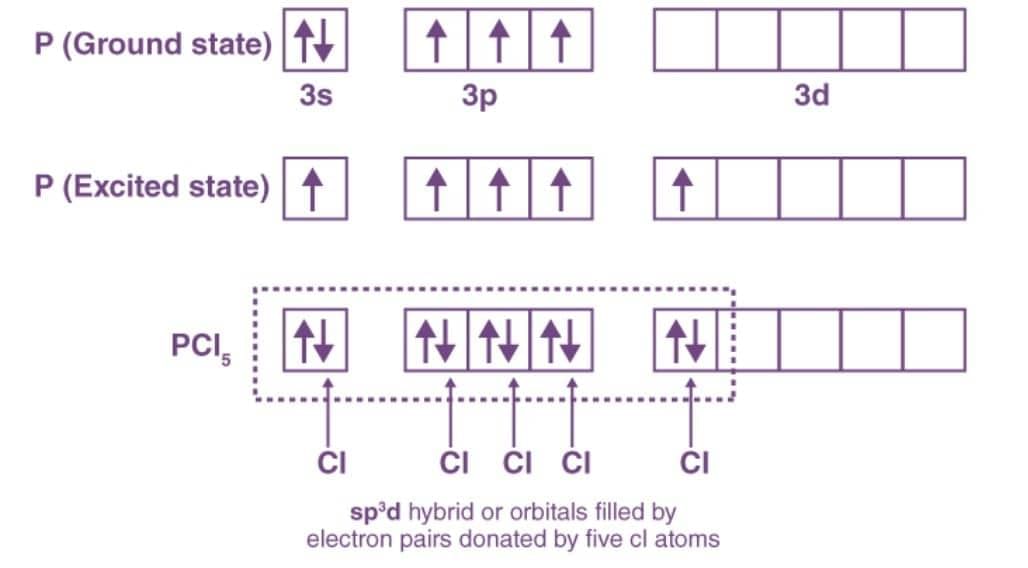
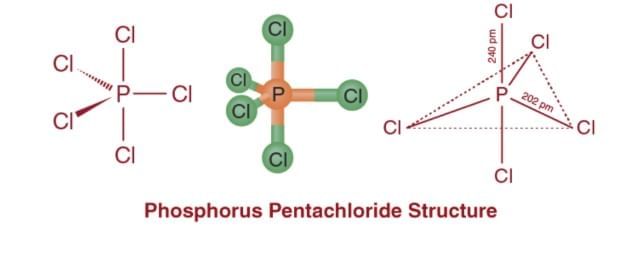
Three P – Cl bonds are equatorial bonds because they are in the same plane and form a 120° angle with each other. The remaining two P – Cl bonds, one above and one below the equatorial plane, form a 90° angle with the plane. These are known as axial bonds. Because axial bond pairs experience more repulsive interaction than equatorial bond pairs, axial bonds have been found to be slightly longer and thus slightly weaker than equatorial bonds, making PCl5 molecules more reactive.
Since SF6 has (sp3d2) hybridization and an octahedral shape with bonds at 90° angles, all of the bonds in SF6 have the same bond length. As a result, the repulsion between all of the bonds is equal.
Q3: Discuss the concept of hybridization. What are its different types in a carbon atom?
Ans: The term “hybridization” was introduced by Pauling. The atomic orbitals, according to him, combine to form a new set of equivalent orbitals known as hybrid orbitals. In contrast to pure orbitals, hybrid orbitals are used in bond formation. The phenomenon is known as hybridization, which is defined as the process of intermixing orbitals with slightly different energies in order to redistribute their energies, resulting in the formation of a new set of orbitals with equivalent energies and shape.
For example, when one 2s and three 2p-orbitals of carbon hybridise, four new sp3 hybrid orbitals are formed.
The following are the main characteristics of hybridization:
- The number of hybrid orbitals equals the number of atomic orbitals hybridised.
- The hybridised orbitals are always energy and shape equivalent.
- Hybrid orbitals are more effective than pure atomic orbitals at forming stable bonds.
- These hybrid orbitals are oriented in space in a preferred direction to achieve the least amount of repulsion between electron pairs and thus a stable arrangement.
As a result, the type of hybridization indicates the molecule’s geometry.
- In carbon compounds, if carbon is linked to carbon via a triple bond, such as alkynes, triple bonded carbon is sp hybridised.
- In carbon compounds, if carbon is linked to carbon via a double bond (C=C), such as in alkenes, the double-bonded carbon is sp2 hybridised.
- In carbon compounds, if carbon is linked to carbon via a single bond (C – C), such as alkanes, the carbon is sp3 hybridised.
|
114 videos|263 docs|74 tests
|
FAQs on Short & Long Question Answers:: Chemical Bonding and Molecular Structure - Chemistry Class 11 - NEET
| 1. What are the main types of chemical bonds and how do they differ? |  |
| 2. How does the concept of electronegativity influence bonding? |  |
| 3. What is the significance of molecular geometry in chemical bonding? |  |
| 4. Can you explain hybridization and its role in bonding? |  |
| 5. What are resonance structures and why are they important in understanding chemical bonds? |  |
















