NEET Exam > NEET Notes > Chemistry Class 11 > Infographic: Structure of Atom
Infographic: Structure of Atom | Chemistry Class 11 - NEET PDF Download
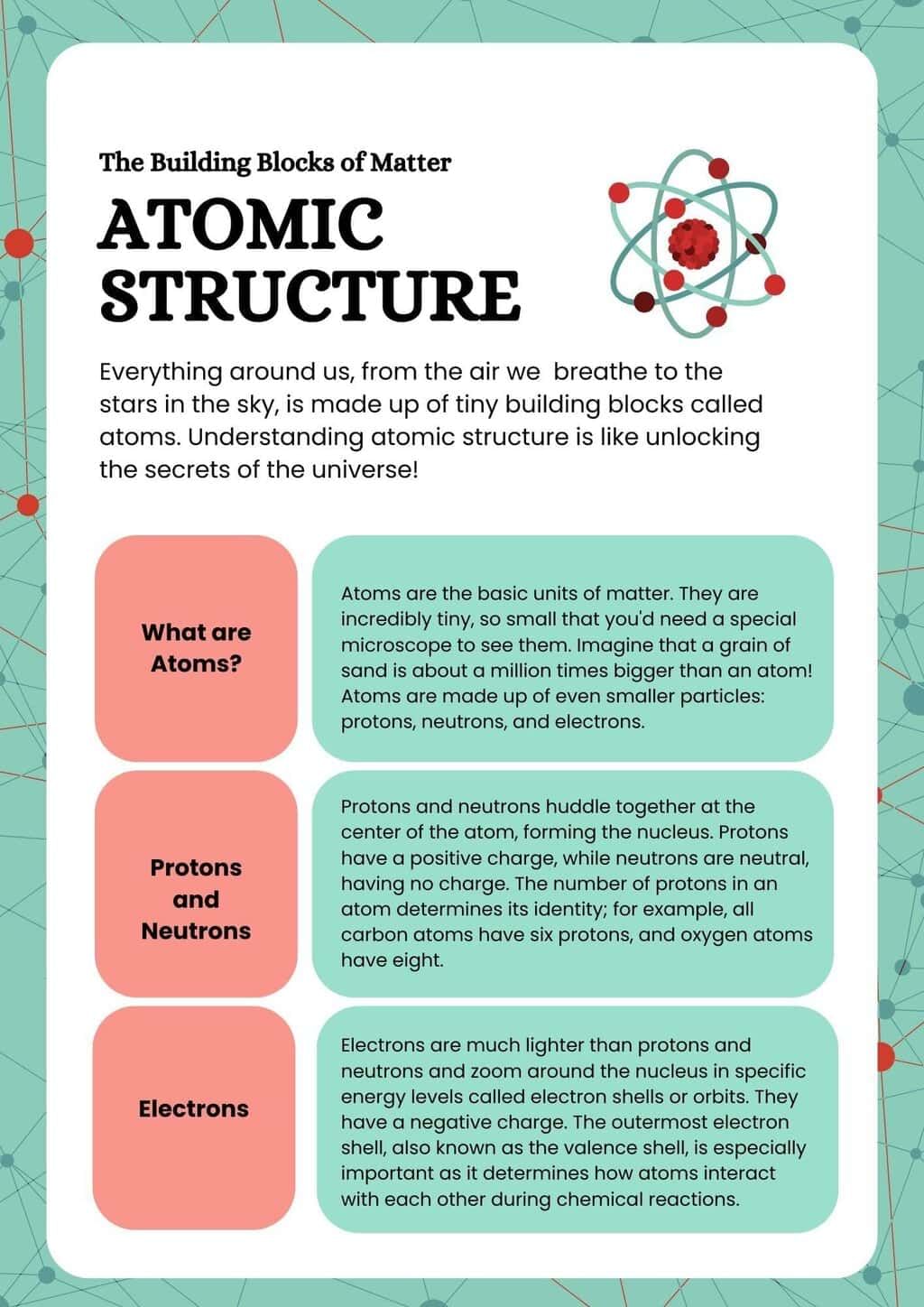

The document Infographic: Structure of Atom | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Infographic: Structure of Atom - Chemistry Class 11 - NEET
| 1. What are the main components of an atom? |  |
Ans. An atom consists of three primary subatomic particles: protons, neutrons, and electrons. Protons are positively charged particles located in the nucleus of the atom, while neutrons are neutral particles that also reside in the nucleus. Electrons are negatively charged particles that orbit the nucleus in various energy levels or shells.
| 2. How do protons and neutrons differ from electrons in terms of mass and charge? |  |
Ans. Protons and neutrons are significantly heavier than electrons. A proton has a mass of approximately 1 atomic mass unit (amu) and carries a positive charge (+1), while a neutron also has a mass of about 1 amu but carries no charge (neutral). In contrast, an electron has a much smaller mass, about 1/1836 of a proton, and carries a negative charge (−1).
| 3. What is the significance of the atomic number and mass number in an atom? |  |
Ans. The atomic number of an atom is defined by the number of protons in its nucleus and determines the element's identity. For example, carbon has an atomic number of 6, meaning it has 6 protons. The mass number, on the other hand, is the sum of protons and neutrons in the nucleus. For instance, a carbon atom with 6 protons and 6 neutrons has a mass number of 12 (6 protons + 6 neutrons = 12).
| 4. How do isotopes of an element differ from one another? |  |
Ans. Isotopes are variants of the same element that have the same number of protons but differ in the number of neutrons. This difference in neutrons results in different mass numbers. For example, ¹²C and ¹³C are isotopes of carbon, with 6 protons and 6 or 7 neutrons, respectively. Isotopes exhibit similar chemical properties but may have different physical properties, such as stability and half-life.
| 5. What role do electrons play in chemical bonding and reactions? |  |
Ans. Electrons, particularly those in the outermost shell (also known as valence electrons), are crucial for chemical bonding. Atoms tend to bond with one another to achieve a stable electron configuration, often through the sharing (covalent bonds) or transfer (ionic bonds) of electrons. The arrangement of electrons in an atom influences its reactivity and the types of chemical reactions it can undergo.
Related Searches
















