NEET Exam > NEET Notes > Chemistry Class 11 > Infographics: Chemical Bonding and Molecular Structure
Infographics: Chemical Bonding and Molecular Structure | Chemistry Class 11 - NEET PDF Download
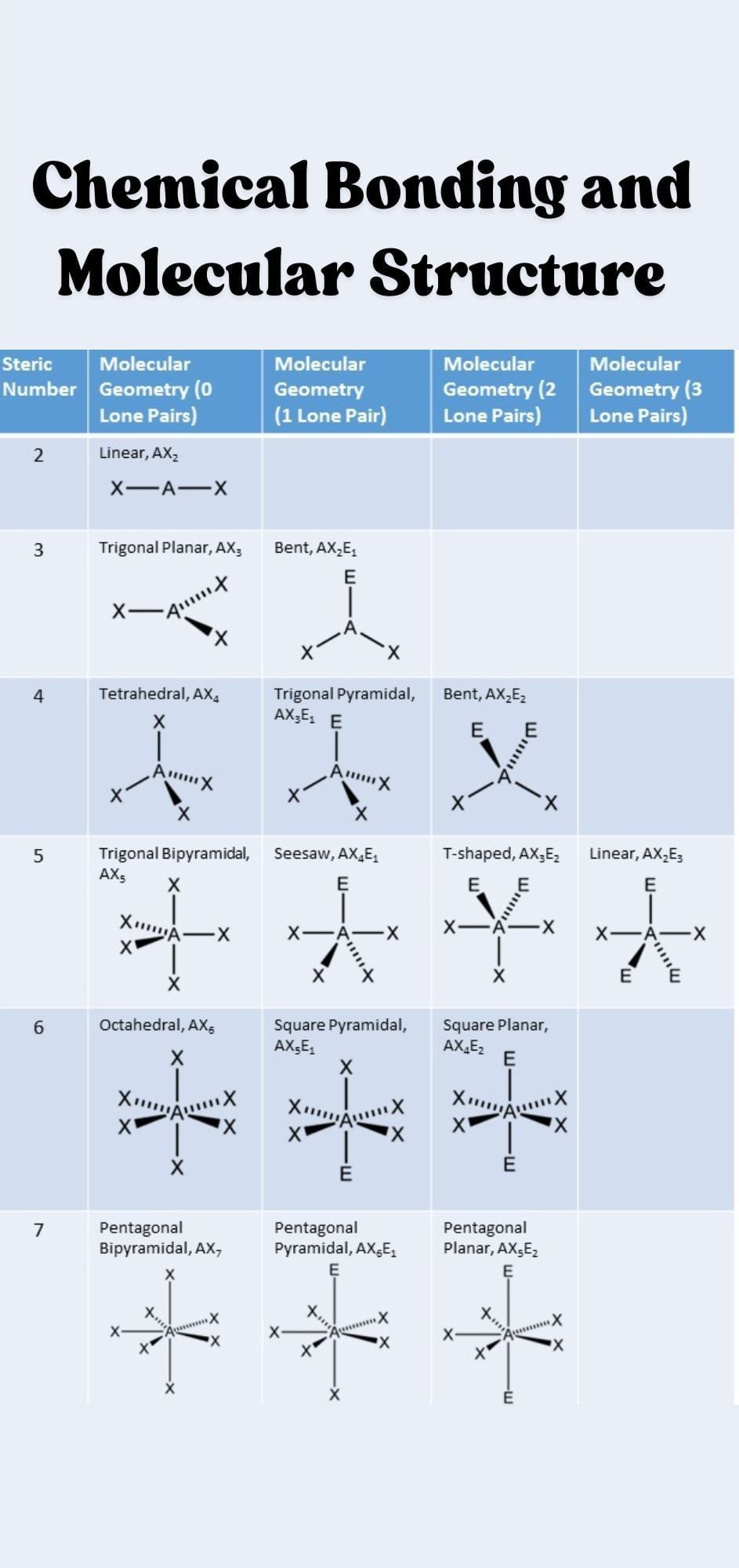
The document Infographics: Chemical Bonding and Molecular Structure | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
121 videos|348 docs|74 tests
|
FAQs on Infographics: Chemical Bonding and Molecular Structure - Chemistry Class 11 - NEET
| 1. What are the different types of chemical bonds and how do they differ? |  |
Ans. The main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. Ionic bonds occur when electrons are transferred from one atom to another, resulting in the formation of ions; for example, Na⁺ and Cl⁻ in NaCl. Covalent bonds involve the sharing of electrons between atoms, such as in H₂O, where hydrogen and oxygen share electrons. Metallic bonds are characterized by a "sea of electrons" that are free to move around, providing metals with their characteristic properties such as conductivity and malleability.
| 2. How does the molecular structure affect the properties of substances? |  |
Ans. The molecular structure, which includes the arrangement of atoms and the types of bonds present, significantly affects the physical and chemical properties of a substance. For instance, the shape of a molecule can influence its polarity, boiling and melting points, and solubility. Polar molecules, like H₂O, tend to have higher boiling points compared to nonpolar molecules of similar size due to stronger intermolecular forces. Additionally, the presence of functional groups can alter reactivity and interaction with other substances.
| 3. What is the VSEPR theory and how is it used to predict molecular shape? |  |
Ans. The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of central atoms. According to VSEPR theory, regions of electron density (bonds and lone pairs) will arrange themselves as far apart as possible to minimize repulsion. For example, a molecule like CH₄ has a tetrahedral shape because the four hydrogen atoms are arranged around the carbon atom at angles of approximately 109.5°.
| 4. What role do electronegativity and polarity play in chemical bonding? |  |
Ans. Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. It plays a crucial role in determining the type of bond formed between atoms. If the difference in electronegativity between two atoms is significant (generally greater than 1.7), an ionic bond is likely to form. If the difference is moderate (between 0.4 and 1.7), a polar covalent bond is formed, resulting in a molecule with partial positive and negative charges. When the difference is small (less than 0.4), the bond is typically nonpolar covalent, with equal sharing of electrons.
| 5. How do resonance structures contribute to the stability of molecules? |  |
Ans. Resonance structures are different ways of drawing the same molecule that represent the delocalization of electrons within it. Molecules with resonance structures often have more stable configurations because the actual structure is a hybrid of all possible resonance forms. For example, in benzene (C₆H₆), resonance structures illustrate that the electrons are delocalized over the entire ring, contributing to its stability and unique properties such as symmetrical bond lengths and high stability against reactions that would disrupt this delocalization.
Related Searches
















