Inside the Atom Chapter Notes | General Science Class 8 (Maharashtra Board) PDF Download
| Table of contents |

|
| Atomic Structure |

|
| Thomson's Model of an Atom |

|
| Rutherford's Model of an Atom |

|
| Bohr's Model of Atom |

|
| Distribution of Electrons in Different Orbits |

|
| Valency |

|
Atomic Structure
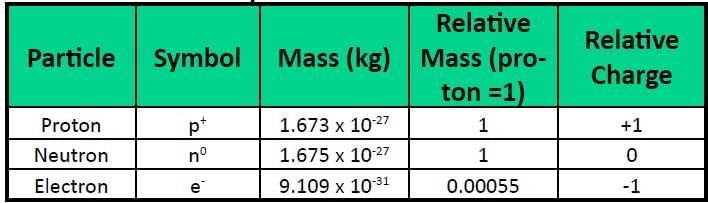
The structure of an atom consists of protons, neutrons, and electrons. Protons and neutrons each have a mass of one unit, while the mass of an electron is so small that it is often ignored. These fundamental components determine the mass and charge of the atom.
Atomic structure is about how these subatomic particles—protons, neutrons, and electrons—are arranged within an atom, which affects its composition and behaviour.
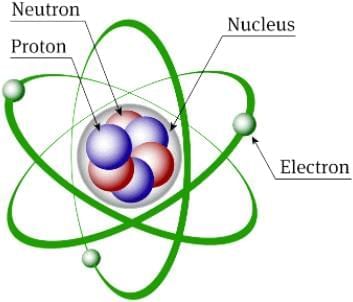
Structure of Atom
- John Dalton believed that the atom cannot be divided.
- In 1866, E. Goldstein found new radiations in a gas discharge tube, naming them canal rays. These rays carry a positive charge.
- In 1897, J.J. Thomson discovered the electron, a subatomic particle with a negative charge.
- The neutron was discovered by Chadwick and has no charge.
Let's Revise: Why is the mass of the electron usually ignored?
 View Answer
View Answer 
Ans: Because it is approximately 1/1836 the mass of a proton, making it negligible.
Thomson's Model of an Atom
Thomson's Model of the Atom, referred to as the plum pudding model, suggested that the atom is made up of a positively charged sphere with negatively charged electrons scattered throughout it, akin to currants in a Christmas pudding. Another way to picture it is like a watermelon, where the positive charge is spread out like the red fruit, and the electrons are like seeds embedded within.
- The electrons are set in a sphere of positive charge, similar to currants in a spherical Christmas pudding.
- The negative and positive charges are balanced, leading to an atom that is overall electrically neutral.
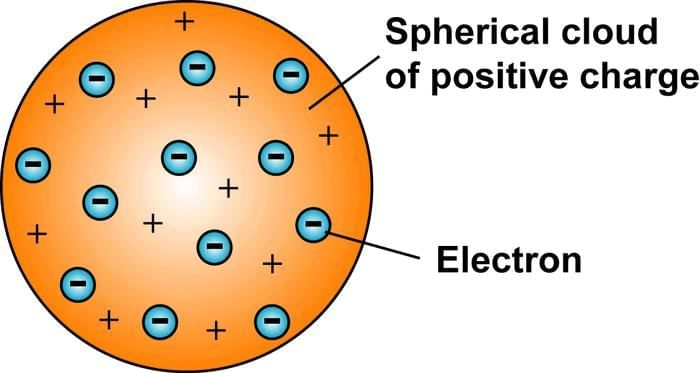 Plum Pudding Model
Plum Pudding Model
[Question: 694264]
Rutherford's Model of an Atom
Rutherford's Model of the Atom brought forth the concept of a small, dense nucleus at the centre of the atom, with electrons moving around it, which greatly changed our understanding of atomic structure.
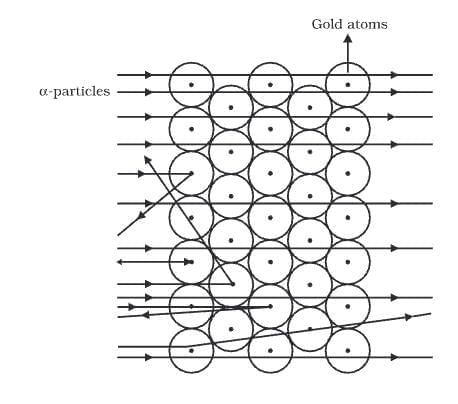 Rutherford's Experiment
Rutherford's Experiment
- α-particles are helium ions with a double charge. Their mass of 4 u gives them significant energy as they move quickly.
- Most of the atom's interior is empty, as many α-particles went through the gold foil without deflecting.
- A few particles were deflected, suggesting that the positive charge of the atom takes up very little space.
- A tiny number of α-particles were deflected back by 180°, showing that the positive charge and mass of the gold atom are concentrated in a very small area.
Conclusions made by Rutherford
- He calculated that the nucleus's radius is about 100,000 times smaller than that of the atom.
- The Nuclear Model of an Atom proposed by Rutherford includes:
- A positively charged centre called the nucleus, where nearly all the mass of the atom is found.
- The electrons orbit the nucleus in circular paths.
- The nucleus is very small compared to the atom's overall size.
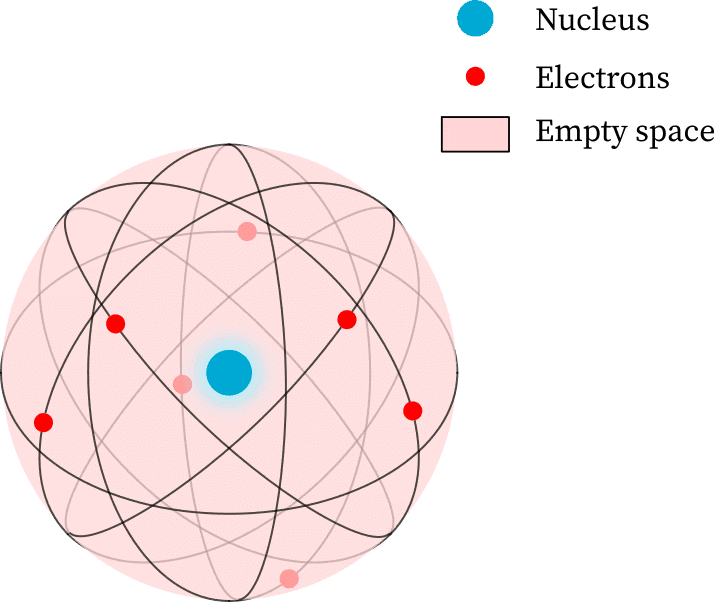 Rutherford's Nuclear Model of Atom
Rutherford's Nuclear Model of Atom
Drawbacks of Rutherford's Model of the Atom
- The orbiting electron in a circular path should not be stable. Any particle in such an orbit would experience acceleration. During this acceleration, charged particles would lose energy by radiating it. Therefore, the electron would lose energy and eventually spiral into the nucleus. If this were true, atoms would be highly unstable, which contradicts the fact that matter exists in a stable form.
Bohr's Model of Atom
Bohr's Model of the Atom changed how we understand atomic structure by introducing the idea that electrons move around the nucleus in specific energy levels. This model helps explain why atoms are stable and how they produce spectral lines.
Historical Context of Niels Bohr
Niels Bohr (1885-1962) was born in Copenhagen on 7 October 1885. He became a professor of physics at Copenhagen University in 1916 and won the Nobel Prize for his contributions to atomic structure in 1922. Some of his important writings include:
- The Theory of Spectra and Atomic Constitution
- Atomic Theory
- The Description of Nature
Postulates of Niels Bohr
- Only certain specific orbits, called discrete orbits, are allowed for electrons inside the atom.
- Electrons do not emit energy while they are moving in these discrete orbits.
- These orbits or shells are referred to as energy levels. Energy levels in an atom are illustrated in Fig. 4.3.
Drawbacks of Bohr's Model of Atom
Despite its significance, Bohr's model has limitations. It contradicts the Heisenberg Uncertainty Principle and fails to explain the spectra of larger atoms. Additionally, it does not fully address atomic stability or the behaviour of electrons in orbits.
Neutrons
In 1932, J. Chadwick discovered a subatomic particle with no charge, which has a mass almost equal to that of a proton. This particle is called a neutron. Neutrons are found in the nucleus of all atoms, except for hydrogen. Generally, a neutron is denoted as 'n'. The mass of an atom is the total of the masses of the protons and neutrons in the nucleus.
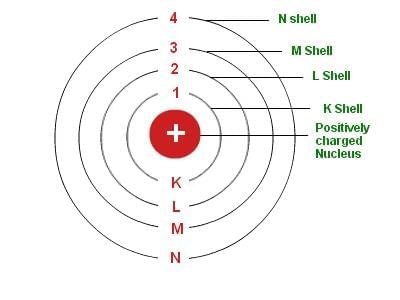 Bohr's Model
Bohr's Model
Let's Revise: How is a hydrogen atom different from atoms of all other elements?
 View Answer
View Answer 
Ans: All atoms consist of three subatomic particles: electrons, protons, and neutrons. The hydrogen atom contains only one electron and one proton, and it has no neutrons, making it unique among all elements.
Distribution of Electrons in Different Orbits
The way electrons are arranged in various orbits, or energy levels, defines an atom's electron configuration.
Rules
- The maximum number of electrons that can fit in a shell is determined by the formula 2n², where 'n' represents the orbit number or energy level index (1, 2, 3, ...).
- The outermost orbit can hold a maximum of 8 electrons.
- Electrons can only fill a shell once the inner shells are filled, meaning shells are filled in a sequential order.
Thus, the maximum number of electrons in various shells is as follows:
- First orbit or K-shell can hold = 2 electrons
- Second orbit or L-shell can hold = 8 electrons
- Third orbit or M-shell can hold = 18 electrons
- Fourth orbit or N-shell can hold = 32 electrons
The atomic structure of the first eighteen elements is illustrated in a diagram.
The electrons in the outermost shell of an atom are called valence electrons. The number of valence electrons is essential in defining the chemical properties of the element.
Valency
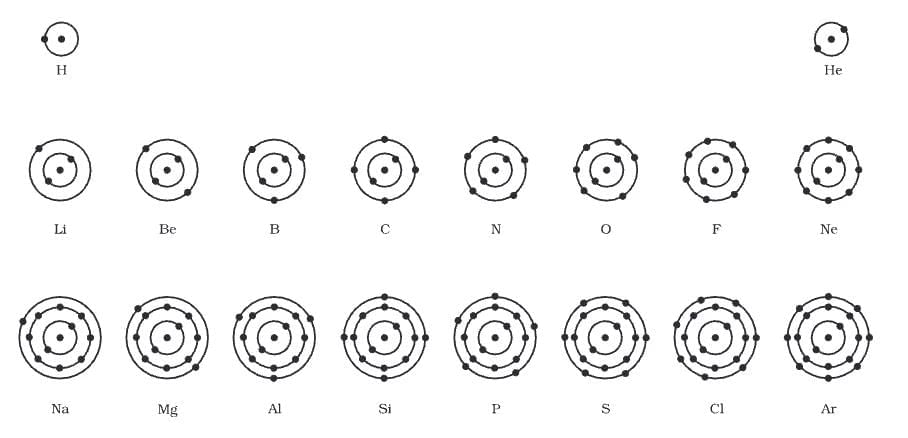 Atomic structure of the first eighteen elements
Atomic structure of the first eighteen elements
- An atom of each element has a definite combining capacity, called its valency.
- The number of bonds that an atom can form in a compound is shown by its valency.
- Valence electrons are the electrons in the outermost orbit of the atom.
Let's Revise
Q: How is the maximum number of electrons in a shell calculated?
 View Answer
View Answer 
Ans: By the formula 2n², where n is the orbit number.
Q: Why are valence electrons important?
 View Answer
View Answer 
Ans: They determine the chemical properties and bonding behaviour of the element.
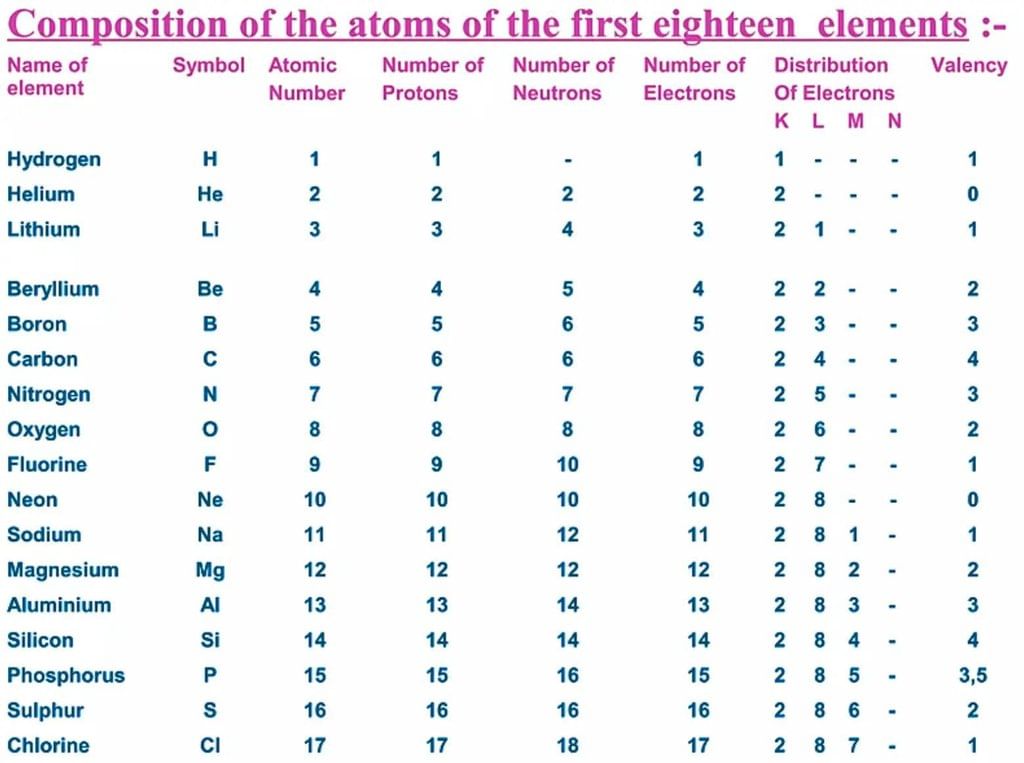
Atomic Number & Mass Number
- The atomic number indicates the number of protons in an atom's nucleus, represented by 'Z'.
- The mass number is the total count of protons and neutrons, giving information about the atom's identity and mass.
- The total number of protons in an atom's nucleus is its atomic number, symbolised as 'Z'.
- The mass number of an atom is the sum of its protons and neutrons, represented by the letter 'A'.
An element is represented as AXZ, where Z is the atomic number (equal to the number of protons), A is the mass number, and X is the element's symbol. The mass number (A) can be calculated as: Mass number (A) = Number of protons (Z) + Number of neutrons.
Let's Revise: What is the mass number?
 View Answer
View Answer 
Ans: The mass number of an element is the total of the number of protons and neutrons in the atom of that element.
Mass Number A = Number of protons + Number of neutrons.
For hydrogen, Z = 1, as there is only one proton in a hydrogen atom's nucleus. Therefore, the mass number of H is 1.
Mass Number A refers to the total count of nucleons (protons and neutrons) in the nucleus.
Isotopes
- Atoms of the same element with the same atomic number but different mass numbers are called isotopes. For example, hydrogen has three isotopes: protium (H), deuterium (²H or D), and tritium (³H or T).
- Chemical properties → same
- Physical properties → different
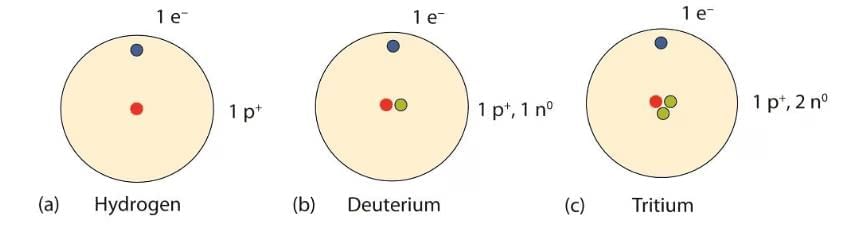 Isotopes of HydrogenApplications of Isotopes:
Isotopes of HydrogenApplications of Isotopes:
(a) An isotope of Uranium is used as fuel in nuclear reactors.
(b) An isotope of Cobalt is used in the treatment of cancer.
(c) An isotope of Iodine is used in the treatment of goitre.
Isobars
- Atoms of different elements that have the same mass number but different atomic numbers are called isobars. For example, carbon-12 and carbon-14 are isobars.
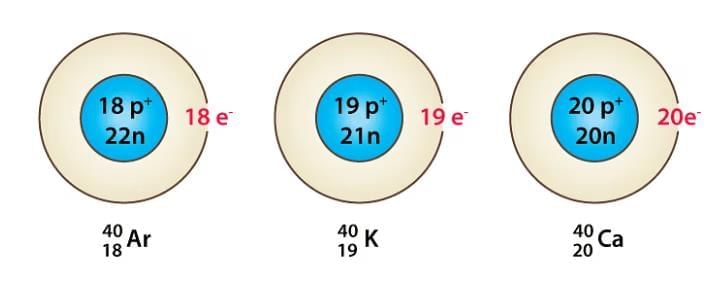 Examples of Isobars
Examples of Isobars
Let's Revise
Q: What are General Features of Isotopes?
 View Answer
View Answer 
- Isotopes of an element have the same atomic number, meaning they have the same number of protons and electrons.
- They have different mass numbers, resulting from a different number of neutrons.
- The chemical properties of isotopes are similar, but their physical properties differ.
- Different masses lead to variations in physical properties like melting point, boiling point, and density.
 View Answer
View Answer 
Example:12C6 and 16O8.
Both C and O have the same number of neutrons i.e. 8.
|
52 videos|175 docs|32 tests
|
FAQs on Inside the Atom Chapter Notes - General Science Class 8 (Maharashtra Board)
| 1. What is Thomson's model of the atom and what were its main features? |  |
| 2. How did Rutherford's model improve upon Thomson's model of the atom? |  |
| 3. What are the key postulates of Bohr's model of the atom? |  |
| 4. How are electrons distributed in different orbits according to Bohr's model? |  |
| 5. What is valency and how is it determined for different elements? |  |














