Cheat Sheet: Carbon and its Compounds - Class 10 PDF Download
Importance of Carbon
Elemental and Combined Forms: Carbon is vital in both elemental (diamond, graphite, fullerenes) and combined forms (e.g., food, clothes, medicines, living structures).
Abundance: Earth's crust contains only 0.02% carbon (minerals like carbonates, coal, petroleum), and the atmosphere has 0.03% carbon dioxide.
Significance: Despite low abundance, carbon’s versatility makes it essential due to its unique properties.
Bonding in Carbon - The Covalent Bond
Carbon’s Electronic Configuration: Atomic number = 6, electron distribution: 1s² 2s² 2p², with 4 valence electrons.
Covalent Bonding: Carbon shares electrons to achieve noble gas configuration (octet), forming covalent bonds instead of gaining/losing electrons (due to high energy requirements for C⁴⁻ or C⁴⁺).
Types of Covalent Bonds:
Single Bond: e.g., Hydrogen (H₂), Methane (CH₄).
Double Bond: e.g., Oxygen (O₂), Ethene (C₂H₄).
Triple Bond: e.g., Nitrogen (N₂), Ethyne (C₂H₂).
Properties of Carbon Compounds:
Low melting and boiling points (e.g., Acetic acid: 290 K melting, 391 K boiling; Methane: 90 K melting, 111 K boiling).
Poor conductors of electricity (no ions formed).
Weak intermolecular forces.
Electron Dot Structures:
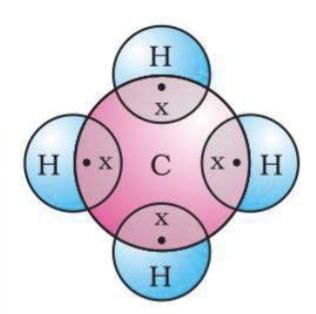
Methane (CH₄): Carbon shares 4 electrons with 4 hydrogen atoms.
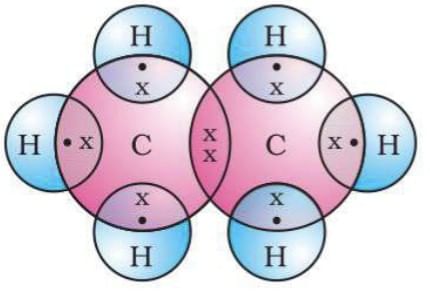
Ethane (CH3-CH3): Each Carbon shares 4 electrons with 3 hydrogen atoms.
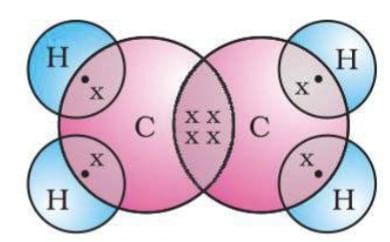
Ethene (CH2-CH2): Each carbon shares double bond with each other and single bond with 2 hydrogen.
Allotropes of Carbon
Diamond: Each carbon bonded to 4 others in a rigid 3D structure; hardest substance, non-conductor.
Graphite: Each carbon bonded to 3 others in hexagonal layers; smooth, slippery, good conductor.
Buckminsterfullerene (C₆₀): Carbon atoms arranged in a football-like structure.
Versatile Nature of Carbon
Catenation: Carbon’s ability to form long chains, branched chains, or rings by bonding with other carbon atoms.
Tetravalency: Carbon’s valency of 4 allows bonding with up to 4 other atoms (carbon or other elements like H, O, N, S, Cl).
Stability: Carbon forms strong bonds due to its small size, making compounds stable.
Organic Compounds:
Initially thought to require a “vital force” (disproved by Wöhler’s synthesis of urea in 1828).
Studied under organic chemistry (except carbides, oxides, carbonates, hydrogencarbonates).
Saturated and Unsaturated Carbon Compounds
1. Saturated Compounds (Alkanes):
Single bonds, less reactive.
Example: Ethane (C₂H₆) – H₃C–CH₃.
2. Unsaturated Compounds:
Alkenes: One or more double bonds, e.g., Ethene (C₂H₄, H₂C=CH₂).
Alkynes: One or more triple bonds, e.g., Ethyne (C₂H₂, HC≡CH).
Chains, Branches, and Rings
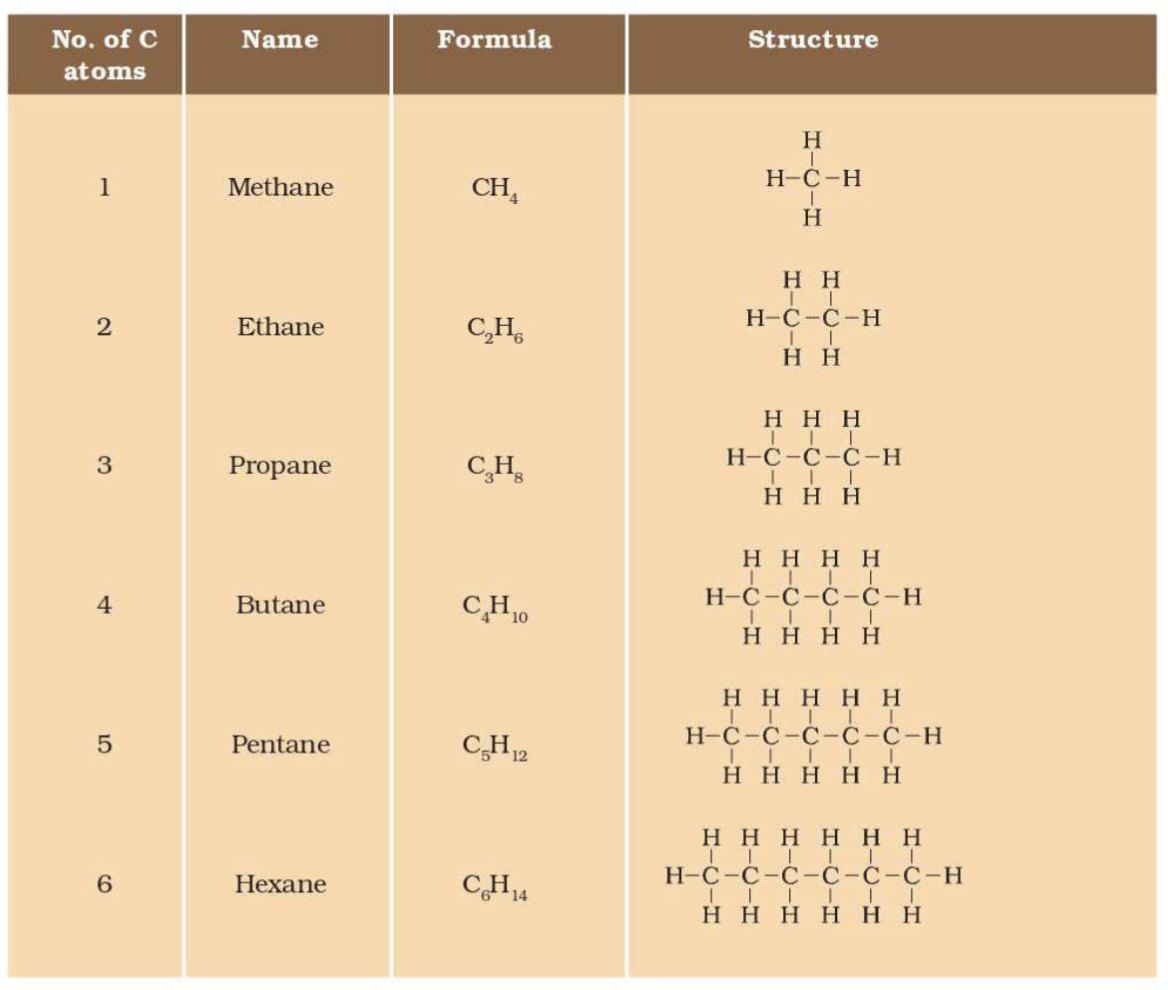
Carbon Chains:
Straight chains: e.g., Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈).
Branched chains: e.g., Isobutane (C₄H₁₀).
Cyclic: e.g., Cyclohexane (C₆H₁₂), Benzene (C₆H₆).
Structural Isomers: Same molecular formula, different structures (e.g., butane vs. isobutane).
Hydrocarbons:
Alkanes: Saturated, single bonds (CₙH₂ₙ₊₂).
Alkenes: One or more double bonds (CₙH₂ₙ).
Alkynes: One or more triple bonds (CₙH₂ₙ₋₂).
Functional Groups
Heteroatoms: Elements like Cl, Br, O, N, S replace hydrogen in hydrocarbons, forming functional groups.
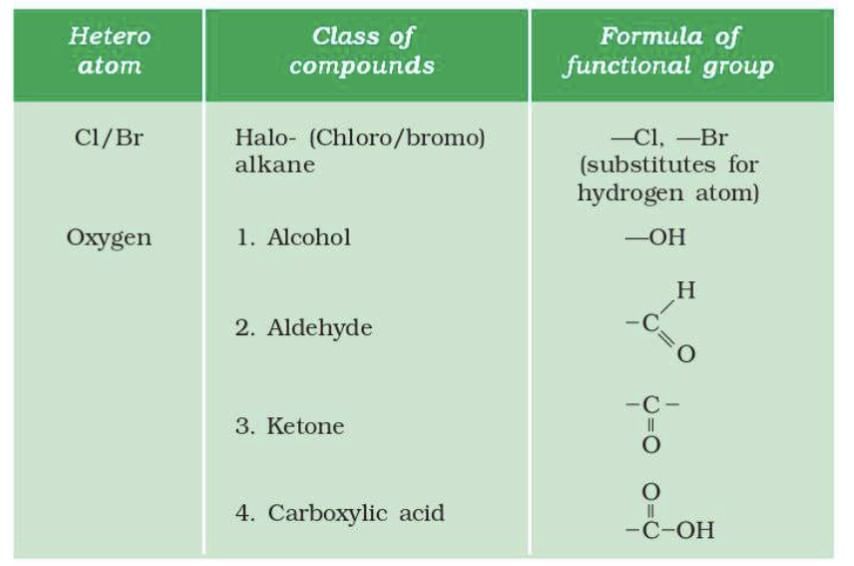
Homologous Series
Series of compounds with the same functional group, differing by a –CH₂– unit.
Example (Alkanes):
Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈), etc.
Differ by –CH₂– units.
Molecular mass difference: 14 u (CH₂ = 12 u (C) + 2×1 u (H)).
Example (Alkenes):
Ethene (C₂H₄), Propene (C₃H₆), Butene (C₄H₈).
Differ by –CH₂– units.
Chemical Properties of Carbon Compounds
1. Combustion: Carbon compounds burn in oxygen to produce CO₂, water, and heat.
Example: CH₄ + 2O₂ → CO₂ + 2H₂O + heat.
Saturated hydrocarbons burn with a clean flame; unsaturated hydrocarbons give a sooty flame due to incomplete combustion.
2. Oxidation: Carbon compounds (e.g., alcohols) can be oxidized to carboxylic acids using oxidizing agents like alkaline KMnO₄ or acidified K₂Cr₂O₇.
Example: Ethanol (C₂H₅OH) → Ethanoic acid (CH₃COOH).
3. Addition Reactions: Unsaturated hydrocarbons undergo addition reactions to become saturated.
Example: Ethene (C₂H₄) + H₂ → Ethane (C₂H₆) (using Ni catalyst).
4. Substitution Reactions: Saturated hydrocarbons (e.g., alkanes) undergo substitution with halogens (e.g., Cl₂) in sunlight.
Example: CH₄ + Cl₂ → CH₃Cl + HCl.
Some Important Carbon Compounds – Ethanol and Ethanoic Acid
1. Ethanol (C₂H₅OH):
Properties: Colorless, pleasant smell, volatile, soluble in water, flammable.
Reactions:
Combustion: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O.
Oxidation: C₂H₅OH → CH₃COOH (with KMnO₄ or K₂Cr₂O�7).
Dehydration: C₂H₅OH → C₂H₄ + H₂O (with conc. H₂SO₄ at 170°C).
Uses: Alcoholic beverages, solvent, fuel, antiseptic.
2. Ethanoic Acid (CH₃COOH):
Properties: Colorless, pungent smell, miscible with water, weak acid.
Reactions:
With NaOH: CH₃COOH + NaOH → CH₃COONa + H₂O.
With NaHCO₃: CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂ (test for carboxylic acids).
Esterification: CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ (ethyl ethanoate) + H₂O.
Uses: Vinegar (5-8% solution), solvent, preservative.
Soaps and Detergents
Soaps:
Sodium or potassium salts of long-chain carboxylic acids (e.g., sodium stearate, C₁₇H₃₅COONa).
Structure: Hydrophilic head (–COO⁻Na⁺) and hydrophobic tail (long hydrocarbon chain).
Cleansing Action: Forms micelles, trapping dirt/oil in the hydrophobic center, allowing it to be rinsed away by water.
Limitation: Ineffective in hard water due to formation of insoluble calcium/magnesium salts (scum).
Detergents:
Synthetic compounds (e.g., sodium alkyl sulphates or sulphonates).
Effective in hard water, no scum formation.
Advantage: Better cleansing in hard water; biodegradable options available.
FAQs on Cheat Sheet: Carbon and its Compounds - Class 10
| 1. Why is carbon known as the backbone of organic chemistry? |  |
| 2. What are the different types of covalent bonds that carbon can form? |  |
| 3. How does carbon bonding affect the physical properties of its compounds? |  |
| 4. What is the significance of hybridization in carbon compounds? |  |
| 5. Why are carbon compounds essential for life? |  |















