Thermal Energy Conductivity Chapter Notes | Science for Grade 6 PDF Download
Introduction
The chapter "Thermal Energy Conductivity" explores how heat, or thermal energy, moves through different materials used in kitchenware. You will learn why some materials heat up or cool down faster than others and how properties like mass, material type, and shape affect this process. This chapter uses fun examples, like cooking pots and baking pans, to explain how thermal energy works in everyday life. By understanding these concepts, you’ll see why certain materials are chosen for things like skillets or hot mitts and how they help in cooking or keeping things cool.
How does mass affect the change in temperature of a substance?
- Mass is the amount of material in an object, like water in a pot.
- More mass means more particles, which need more energy to heat up.
- If you heat two pots with different amounts of water, the one with less water heats up faster.
- This is because less mass needs less energy to reach the same temperature.
- For example, a small pot of water boils faster than a large pot with the same heat.
- The relationship between mass and temperature change is inversely proportional: as mass increases, the temperature change decreases for the same heat input.
How does the type of matter affect the change in temperature of a substance?
- Different materials, like metal, cotton, or glass, transfer heat in different ways.
- Metal pans heat up quickly because they are good at transferring heat.
- Cotton or silicone hot mitts don’t let heat pass through easily, so they stay cooler.
- The type of material decides how fast or slow heat moves through it.
Materials and Energy Transfer
- Materials like aluminum, cotton, and air transfer heat differently.
- Aluminum lets heat pass through quickly, making it good for cooking pans.
- Cotton and air block heat, so they are used in things like hot mitts or insulation.
- The amount of heat a material transfers depends on its properties.
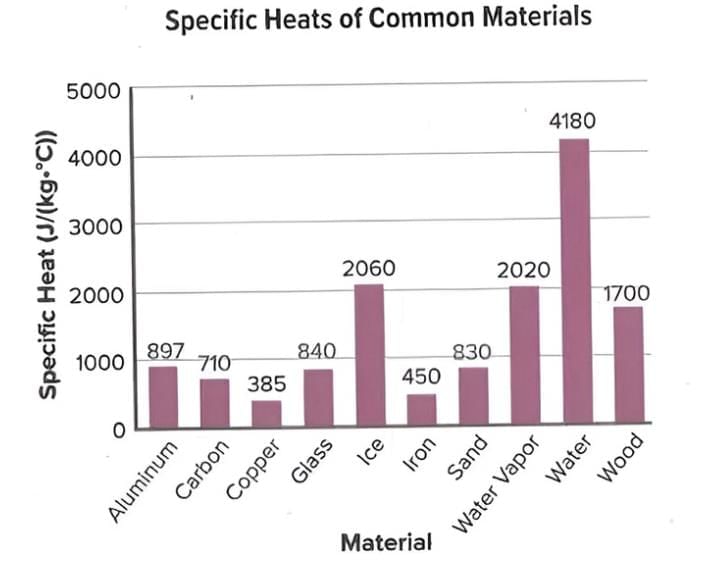
Specific Heat
- Specific heat is the amount of energy needed to raise the temperature of 1 kg of a material by 1°C.
- Materials with low specific heat, like metals, heat up quickly with less energy.
- Materials with high specific heat, like water, need more energy to heat up.
- Water has a high specific heat, so it takes a lot of energy to change its temperature.
- This is why pools, lakes, and oceans stay cool in summer and warm in winter.
- Areas near large bodies of water have milder climates because water’s high specific heat keeps temperatures steady.
Conductors and Insulators
- Materials are divided into thermal conductors and thermal insulators based on how they transfer heat.
- Thermal conductors let heat flow easily because their particles move energy quickly.
- Metals, like iron or aluminum, are good thermal conductors.
- Thermal insulators block heat flow because their particles don’t transfer energy easily.
- Nonmetals, like wood or cotton, are good thermal insulators.
- For example, a pan’s iron body conducts heat well, but its wooden handle stays cool because it’s an insulator.
- Conductors have lower specific heats, so they need less energy to heat up.
- Insulators have higher specific heats, so they need more energy to change temperature.
- Metal pans heat up faster than glass pans, but glass cools down slower.
Did You Know?
Water has a very high specific heat, which means it takes a lot of energy to raise its temperature by just 1°C. This special property helps keep pools, lakes, and oceans cool during the summer. It also affects nearby land areas, giving them more moderate climates. Places near large bodies of water are usually cooler in the summer and warmer in the winter because water heats up and cools down slowly!
What other properties affect thermal energy transfer?
- Besides mass and material type, other properties like shape, thickness, and color affect heat transfer.
- Kitchenware comes in different shapes, like thick cast iron skillets or thin aluminum pans.
- These properties change how fast or slow heat moves through the material.
Thermal Energy and Properties of Materials
Different properties of a substance can influence how thermal energy is transferred. These properties include how reflective the material is, how thick it is, and how much surface area it has.
Reflectivity v. Absorbency
- Reflectivity is when energy bounces off a surface, like light off a mirror.
- Absorbency is when a material takes in energy, like heat.
- White surfaces reflect light and heat, staying cooler.
- Black surfaces absorb light and heat, getting warmer faster.
- For example, a white plate stays cooler than a black one in the sun.
Thickness
- Thicker materials take longer to heat up because heat travels a longer distance.
- Thick materials also cool down slower.
- For example, a thick cast iron skillet heats up slower than a thin aluminum pan.
- Thickness is related to mass; thicker objects usually have more mass.
Surface Area
- Surface area is the outer area of an object exposed to the environment.
- More surface area means more heat transfer to or from the surroundings.
- For example, a shallow bowl loses heat faster than a deep bowl because it has more surface area.
- Objects with larger surface areas cool down or heat up faster.
A Closer Look: Heat Sinks
- Heat sinks are objects designed to absorb and spread out heat.
- They are often made of materials like aluminum or copper, which are good conductors.
- Heat sinks have large surface areas to transfer heat to the surroundings quickly.
- In kitchens, metal pans act like heat sinks, spreading heat evenly to cook food.
|
252 docs|10 tests
|




















