Particulate Nature of Matter Chapter Notes | Science Curiosity Class 8 - New NCERT PDF Download
Why can you stack a pile of sand, but not a puddle of water? This chapter will take you on a journey into the tiny building blocks that make up everything around us. You'll find out what matter really is? Why solids, liquids, and gases behave so differently, and just how small the basic particles of matter can be!
What Is Matter Composed of?
All matter is made up of tiny units called constituent particles or basic building blocks.
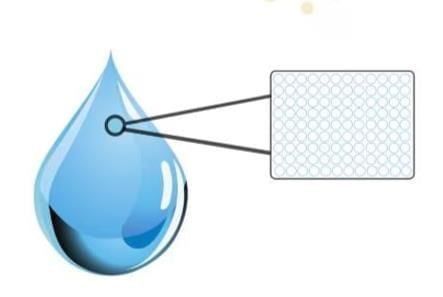 Matter is made up of tiny particles
Matter is made up of tiny particles
Example 1: Breaking Down Chalk
- Breaking a piece of chalk into two… then into smaller and smaller pieces… and finally grinding it into fine powder.
- Even the finest chalk powder you can make still looks like chalk under a magnifying glass.
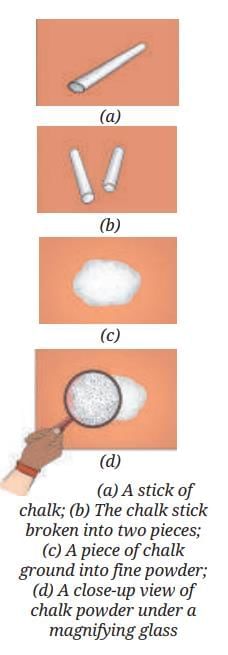
- This shows that no matter how small the pieces become, each is still chalk. Breaking or grinding chalk does not change it into something else; it is still the same substance.
- Grinding is a physical change—no new substance is formed; only the size of each piece changes.
- If you could keep breaking the chalk smaller and smaller (far beyond what you can see), you’d eventually reach the constituent particles that can’t be broken down further by normal means.
- Constituent Particle: The constituent particle is the basic unit that makes up a substance.
Example 2: Dissolving sugar in water
- When sugar is added to water (without stirring), the top layer does not taste sweet.
- After stirring, the whole solution tastes sweet—even though you cannot see any sugar grains.

- The sugar has disappeared from sight, but you can taste it—sugar particles are still present, just too small to be seen.
- The sugar has separated into its constituent particles, which spread out among the water particles and occupy the available spaces (called interparticle spaces).
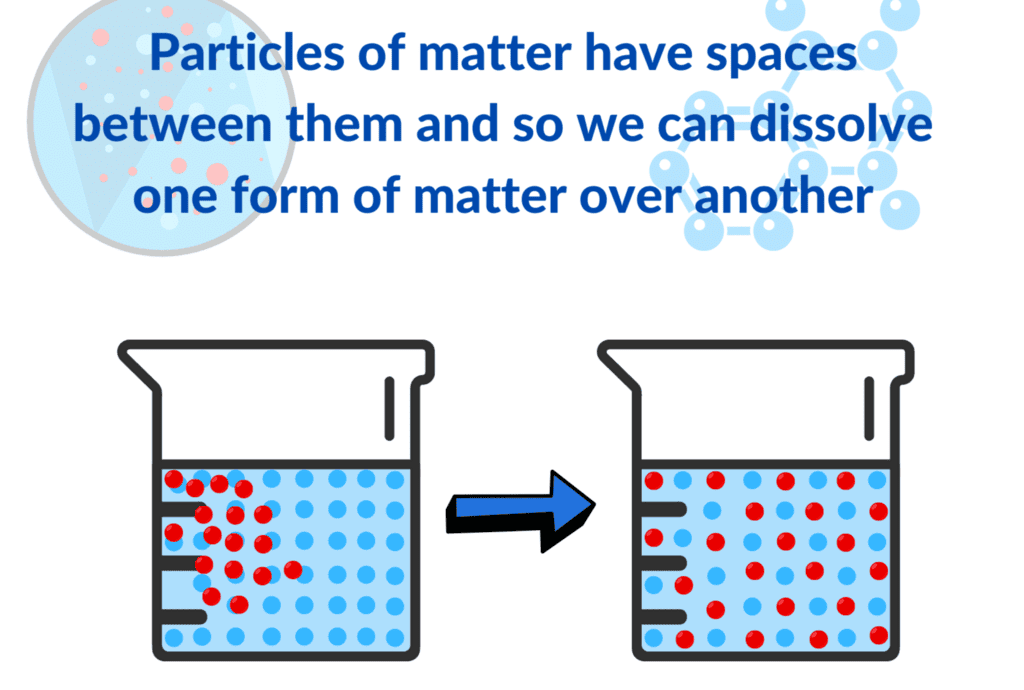
Conclusion:
Both chalk and sugar can be broken down to pieces made of their basic particles, and these are so small they are invisible to the naked eye or even ordinary microscopes.
What Decides Different States of Matter?
The constituent particles of matter are held together through forces which are attractive in nature. These forces are called interparticle attractions
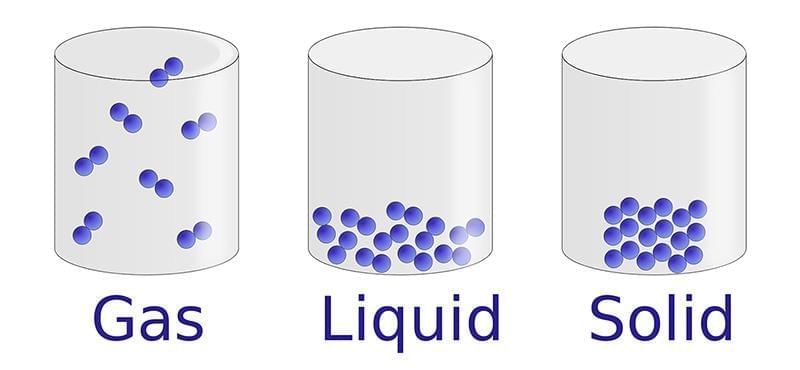 Interparticle Attractions in Matter
Interparticle Attractions in Matter
- The strength of these inter attractions depends on how close the particles are and the nature of the substance.
- The physical state (solid, liquid, or gas) depends on:
- The strength of the interparticle attractions.
- The distance between the particles.
Solid State
In solids, particles are packed tightly together. The interparticle attractions are very strong, so particles cannot move freely—they just vibrate about their positions.

Why are solids hard and keep their shape?
When you try to hammer objects like an iron nail, piece of wood, or rock salt, they all:
- Have a definite shape and volume. This is due to the fact that in solids, the particles are tightly packed and the interparticle attractions are very strong.
- These strong forces of attraction keep the particles in solids in fixed positions, giving solids a definite shape and making them hard to break apart.
When Solids Melt:
- When solids are heated, their particles vibrate more vigorously.
- A stage is reached when these vibrations become so vigorous that the particles start leaving their position.
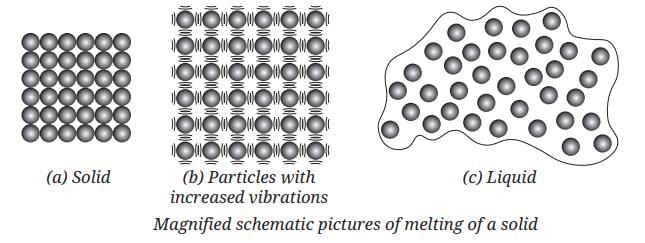
- The interparticle forces of attraction get weakened and the solid gets converted into the liquid state
- The temperature at which this happens is the melting point of the solid.
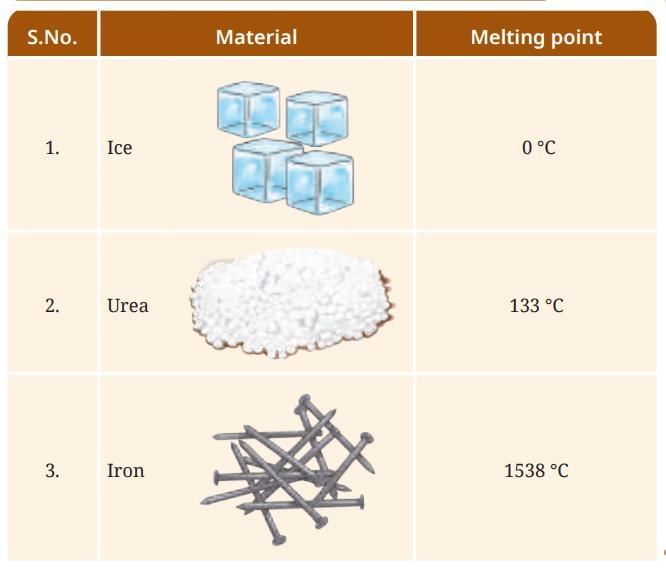
Melting Point: The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point. Some examples of solids and their melting points are:
Liquid State
In liquids, particles are less tightly packed than in solids. They are still close together, but interparticle attractions are weaker than in solids.
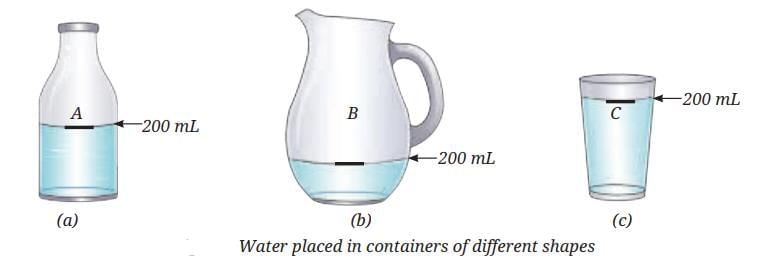
Why do liquids flow and take the shape of containers?
When water is poured from one container to another:
- The water always takes the shape of the container.
- The volume stays the same.
- Liquid particles can move past each other, so liquids do not have fixed shapes but have a fixed volume.
- You can move your finger through water because its particles are not fixed—they are free to move, but only within the volume of the liquid.
Heating Liquids
When a liquid is heated, its particles gain energy, leading to increased movement.
Eventually, a stage is reached where the liquid starts boiling, marking the rapid conversion to vapor.
Key Definitions
Boiling Point: The specific temperature at which a liquid boils and turns into vapor under atmospheric pressure. At this point, particle movement becomes extremely vigorous, causing particles to move apart and weakening the interparticle forces of attraction.
Boiling: The fast process of vapor formation that occurs at the boiling point, happening not only at the liquid's surface but also within the liquid itself. This is visible as bubble formation, as constituent particles escape the liquid state and convert it into vapor (gaseous state).
Evaporation: The slower process of vapor formation that occurs at all temperatures, even below the boiling point. Unlike boiling, it happens only at the liquid's surface and proceeds gradually without bubble formation.
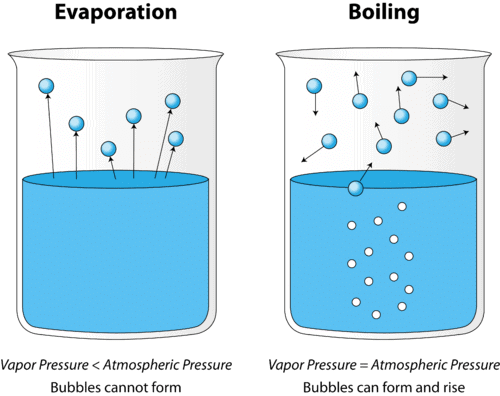
Particle Behavior During Heating
As heating continues, particles move more vigorously and separate from each other.
This separation results in a decrease in interparticle forces of attraction, allowing particles to escape the liquid and form vapor.
The overall transformation: The liquid converts into its gaseous state (vapor), with boiling being the rapid, internal version and evaporation the slower, surface-only version.
Gaseous State
In gases, particles are very far apart and interparticle attractions are almost zero. Gas particles can move freely in all directions.
Why do gases have no fixed shape or volume?
- When you trap smoke in a glass jar and then allow it to move into a second jar:
- The smoke spreads and fills the entire space of the new jar.
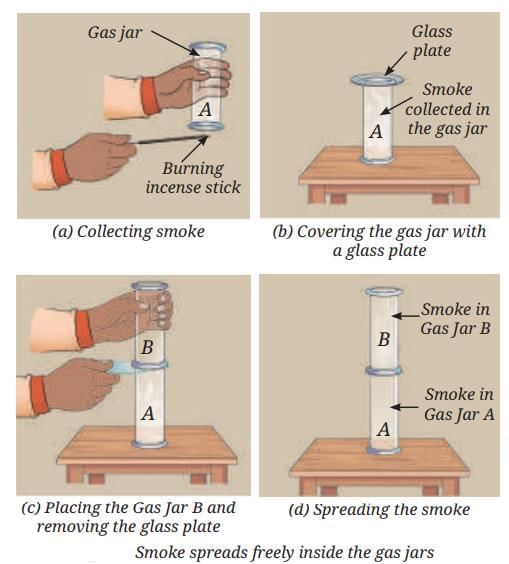
- Gas particles always spread out to completely fill any container.
- Gases do not have a fixed shape or a fixed volume—they are compressible and can expand to fill any space.
- This is seen with both smoke and iodine vapour (or any gas)—particles are always in rapid, random motion.

Summary of the Section:
- Solids: Particles are packed tightly due to strong attractions—giving definite shape and volume.
- Liquids: Particles can move past each other; weaker attractions let them flow and take the container’s shape, but not compress much.
- Gases: Particles are far apart with negligible attractions; no definite shape or volume, can fill any container, highly compressible.
How Does the Interparticle Spacing Differ in the Three States of Matter?
The spaces between constituent particles—called interparticle spacing—play a crucial role in determining whether a substance is a solid, liquid, or gas.
- Smaller interparticle spacing: Stronger attractions; less movement; substance is more rigid (solid).
- Larger interparticle spacing: Weaker attractions; more movement; substance is fluid or gaseous.
Understanding Compression in Fluids (Gas and Liquid) using a Syringe
- When you pull the plunger of a syringe, air fills the inside. Put your thumb over the open end and push the plunger in—the volume of air decreases.
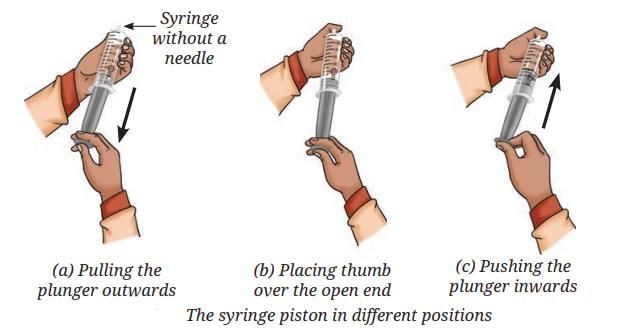
- Gas particles have large gaps between them, so gases are easily compressible. When compressed, particles are forced closer together.
- Repeat the experiment with water: Try pushing the plunger when the syringe is filled with water (thumb on end). The plunger cannot move much.
- Conclusion: Liquids have much smaller gaps between particles, so they are almost incompressible.
Dissolving Sugar—Evidencing Interparticle Spaces in Liquids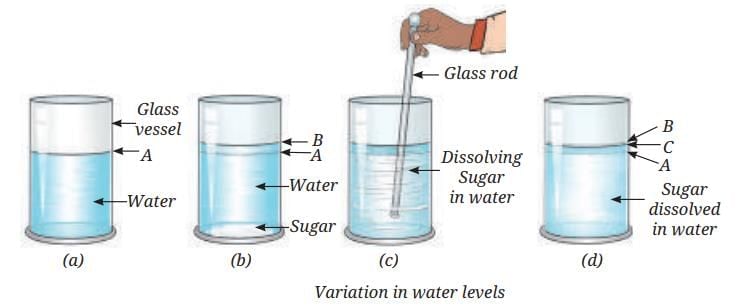
Add sugar to water in a glass vessel:
- Water level first rises (as sugar is added).
- After stirring, sugar dissolves; the final liquid level (C) is less than the expected sum of water plus sugar.
- There are empty spaces between water particles (interparticle spaces). When sugar dissolves, its particles fill these spaces.
- If you add an insoluble solid (like sand), the water level stays high or rises, because sand does not dissolve or fill the interparticle spaces—the particles of sand just add their own volume.
What About Solids?
- In solids, strong attractions hold the particles tightly together in a fixed arrangement.
- Interparticle spacing is very small—the particles are closely packed with little movement.
- Even though they’re tightly packed, a tiny amount of space still exists between the particles, but it’s much less than in liquids or gases.
- These spaces contain nothing (not even air).
Scientific Meaning of “Particle”
- In science, “particle” can mean the constituent unit that builds matter (atoms or molecules)—these are much, much smaller than dust or sand grains.
- Other contexts (like air pollution) use "particle" for small dust bits, but even those are made of atoms/molecules.
Summary of the section
- Gases: Can be compressed easily—lots of space between particles.
- Liquids: Nearly incompressible—small interparticle gaps; dissolved substances can fit into these spaces.
- Solids: Fixed, small gaps—particles are packed tightly, hardly any compressibility.
How Particles Move in Different States of Matter
Particles Are Always in Motion: The movement of particles (tiny basic units) is a key reason for differences in the behavior of solids, liquids, and gases.
Drop potassium permanganate in water:
- At first, you see colored streaks spreading out.
- Soon, the whole glass of water is evenly colored.
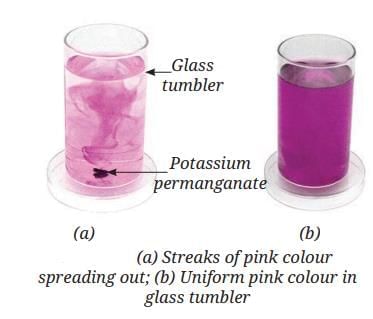
- Reason: The water particles are constantly moving. They pull potassium permanganate particles from the grain, and then both kinds of particles keep moving and mixing until the color is uniform.
- Key Concept: Particles of liquids are always moving, pulling apart and mixing other particles—this is why substances can dissolve and diffuse in water.
How Temperature Affects Movement:
- The color spreads faster in hot water, slower at room temperature, slowest in ice-cold water.
- More heat energy = faster movement of particles.
Movement in Gases (Incense Stick Experiment)
- Burn an incense stick in one corner of a room.
- Soon, the fragrance spreads throughout the room, even to people far from the incense stick.
- Reason: Air particles are always moving rapidly in all directions, colliding with and spreading the fragrance particles.
- Key Concept: Particles of gases are in constant, fast motion, filling all available space and carrying other particles with them (diffusion).
Why Some Substances Don’t Dissolve
Some solids (like sand) don’t dissolve in water. Their particles are held together too tightly and aren’t pulled apart or mixed by the moving water particles.
Other Real-Life Examples of Movement of Gases:
- Cooking Smells: When someone cooks food in the kitchen, you quickly smell it in other rooms, even before you see the food. Smell particles move through the air into every part of the house.
- Gas Leak Detection: If there’s a gas leak or someone breaks open a bottle of ammonia, the sharp smell rapidly spreads, even to people far away.
- Smoke or Fog: If someone smokes or a candle burns, the smoke travels and fills the space, showing how gas particles (and suspended particles) move.
- Air Fresheners: Plug-in or spray air fresheners quickly make a room smell pleasant because their tiny scent molecules travel via moving air particles.
How Soap Cleans Oily Stains (Particulate Nature in Action):
- Soap molecules have two ends: One end sticks to oily stains (which water alone can’t remove).
- The other end mixes with water.
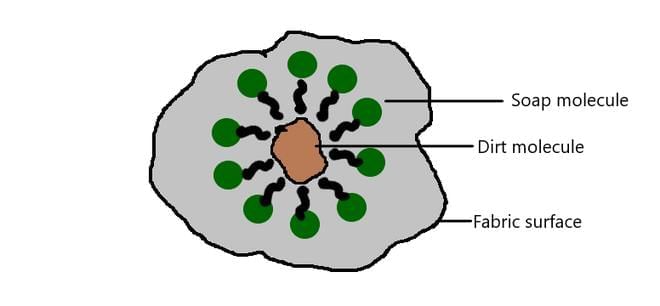
- When soap is added to stained clothes, the soap particles “grab” the oil and let water wash it away, demonstrating how tiny particles (of soap, oil, and water) interact and move.
- This microscopic movement is why washing with soap and water lifts away dirt and oil from clothes and skin.
Thermal Energy and Particle Movement
The thermal (heat) energy of particles decides how much they move and how far apart they are:
- Solids: Lowest thermal energy; particles vibrate in place.
- Liquids: More energy; particles move around each other, but not too far.
- Gases: Highest energy; particles move freely in all directions, far apart.
Summary Table:
Particle nature of the three states of matter–
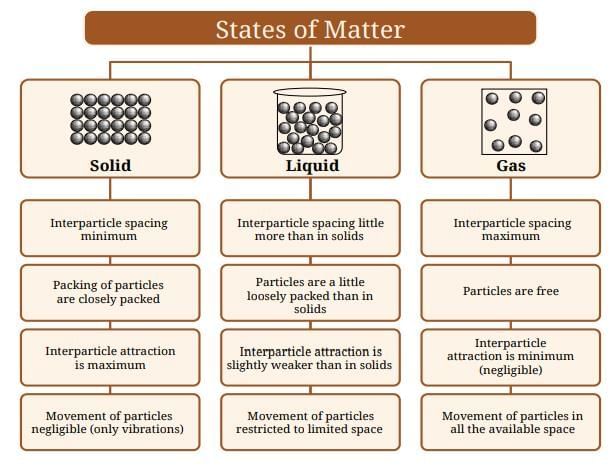
Key Terms to Remember
- Matter: Anything composed of extremely small particles that occupy space and have mass.
- Constituent Particles: The tiny, basic building blocks that make up matter; they are extremely small and cannot be seen with the naked eye.
- Interparticle Forces of Attraction: The attractive forces that hold particles together; strongest in solids, weaker in liquids, and weakest (negligible) in gases.
- Interparticle Spacing: The space or distance between particles; minimum in solids, slightly more in liquids, and maximum in gases.
- Solid State: A state of matter with strong interparticle attractions, minimum spacing, and no free particle movement, resulting in fixed shape and fixed volume (size).
- Liquid State: A state of matter with slightly weaker interparticle attractions than solids, allowing limited particle movement and more spacing; has a definite (fixed) volume but no fixed shape.
- Gaseous State: A state of matter with negligible interparticle attractions, free particle movement, and maximum spacing; has no fixed shape or volume.
- Fixed Shape: A property where matter maintains its form (seen in solids due to strong attractions and minimal spacing); liquids and gases lack this.
- Fixed Volume: A property where matter occupies a definite amount of space (seen in solids and liquids); gases do not have this as they expand to fill available space.
- Particle Movement: The motion of particles; none (only vibrations) in solids, limited in liquids, and completely free in gases, influenced by interparticle attractions and spacing.
|
54 videos|263 docs|13 tests
|
FAQs on Particulate Nature of Matter Chapter Notes - Science Curiosity Class 8 - New NCERT
| 1. What is matter composed of? |  |
| 2. How does interparticle spacing differ in the three states of matter? |  |
| 3. How do particles move in different states of matter? |  |
| 4. Why are solids rigid while liquids and gases are not? |  |
| 5. What role does temperature play in the state of matter? |  |





















