Previous Year Questions: Metals and Non-metals - Class 10 PDF Download
Q1: The metals obtained from their molten chlorides by the process of electrolytic reduction are:
(a) Gold and silver
(b) Calcium and magnesium
(c) Aluminium and silver
(d) Sodium and iron
 View Answer
View Answer 
Ans: (b) Calcium and magnesium
Explanation:
- Highly reactive metals like sodium, calcium, magnesium, and aluminium are extracted by electrolytic reduction of their molten chlorides.
- Gold and silver are less reactive, often found native or extracted by other methods (e.g., cyanide process).
- Iron is extracted by reduction with carbon in a blast furnace.
- Calcium and magnesium fit the description as they require electrolysis (e.g., NaCl or CaCl₂).
Q2: The formation of magnesium oxide is correctly shown in option:
(a) Mg · O → Mg²⁺[O²⁻]
(b) Mg · O → Mg⁺[O²⁻]
(c) Mg · O → Mg²⁺[O²⁻]₂
(d) 2Mg × O → [Mg²⁺]₂[O²⁻]
 View Answer
View Answer 
Ans: (a) Mg · O → Mg²⁺[O²⁻]
Explanation:
- Magnesium (Mg, atomic number 12: 2,8,2) loses 2 electrons to form Mg²⁺.
- Oxygen (O, atomic number 8: 2,6) gains 2 electrons to form O²⁻.
- The ionic compound is MgO (Mg²⁺[O²⁻]), with a 1:1 ratio.
- Option (a) correctly shows the electron transfer and ionic bond formation.
Q3: The most common method of extraction of metals from their oxide ores is:
(a) Reduction with carbon
(b) Reduction with hydrogen
(c) Reduction with aluminium
(d) Electrolytic reduction
 View Answer
View Answer 
Ans: (a) Reduction with carbon
Explanation:
- Metals in the middle of the reactivity series (e.g., iron, zinc) are commonly extracted by reducing their oxides with carbon
(e.g., Fe₂O₃ + 3C → 2Fe + 3CO). - Hydrogen reduction is less common, used for specific metals like tungsten.
- Aluminium reduction (thermite) is specific to certain metals (e.g., chromium).
- Electrolytic reduction is for highly reactive metals (e.g., sodium, aluminium).
Q4: Reaction between two elements A and B, forms a compound C. A loses electrons and B gains electrons. Which one of the following properties will not be shown by compound C?
(a) It has high melting point.
(b) It is highly soluble in water.
(c) It has weak electrostatic forces of attraction between its oppositely charged ions.
(d) It conducts electricity in its molten state or aqueous solution.
 View Answer
View Answer 
Ans: (c) It has weak electrostatic forces of attraction between its oppositely charged ions.
Compound C is ionic (A loses electrons, B gains, forming A⁺ and B⁻).
Ionic compounds have:
- High melting points due to strong electrostatic forces (a).
- High solubility in water for many ionic compounds (b).
- Conductivity in molten or aqueous state due to mobile ions (d).
- Weak electrostatic forces (c) are not characteristic, as ionic bonds are strong.
Q5: Aluminium powder is used in thermit welding because:
(a) Its reaction with iron is highly exothermic.
(b) When it is heated with iron (III) oxide, molten iron is obtained.
(c) When it is heated with iron (III) oxide, molten aluminium oxide is obtained to join railway tracks.
(d) Its melting point is low as compared to iron and a molten alloy of iron and aluminium is formed on heating which is used to join railway tracks.
 View Answer
View Answer 
Ans: (b) When it is heated with iron (III) oxide, molten iron is obtained.
- Thermite reaction: 2Al + Fe₂O₃ → 2Fe + Al₂O₃ + Energy.
- Aluminium reduces iron oxide to molten iron, which is used to join railway tracks.
- The reaction is exothermic (A is partially correct but less specific).
- Molten aluminium oxide (c) is a byproduct, not used for joining.
- No alloy is formed (D is incorrect).
Q6: The products formed when Aluminium and Magnesium are burnt in the presence of air respectively are:
(a) Al₃O₄ and MgO₂
(b) Al₂O₃ and MgO
(c) Al₃O₄ and MgO
(d) Al₂O₃ and MgO₂
 View Answer
View Answer 
Ans: (b) Al₂O₃ and MgO
- Aluminium burns in air: 4Al + 3O₂ → 2Al₂O₃ (aluminium oxide).
- Magnesium burns in air: 2Mg + O₂ → 2MgO (magnesium oxide).
- Al₃O₄ and MgO₂ are not chemically correct formulas.
Q7: A metal, M, displaces iron from aqueous solution of ferrous sulphate but fails to do so in case of aqueous solution of aluminium sulphate. The metal M is:
(a) Magnesium
(b) Copper
(c) Lead
(d) Zinc
 View Answer
View Answer 
Ans: (d) Zinc
- Reactivity series: K > Na > Ca > Mg > Al > Zn > Fe > Pb > Cu.
- Metal M displaces Fe from FeSO₄, so M is above Fe (Mg, Al, Zn).
- M does not displace Al from Al₂(SO₄)₃, so M is below Al.
- Zinc (Zn) fits: Zn + FeSO₄ → ZnSO₄ + Fe; no reaction with Al₂(SO₄)₃.
Q8: The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
(a) Blue
(b) Pale green
(c) Yellow
(d) Reddish brown
 View Answer
View Answer 
Ans: (b) Pale green
- Reaction: Fe + CuSO₄ → FeSO₄ + Cu.
- CuSO₄ solution is blue (Cu²⁺ ions).
- FeSO₄ solution is pale green (Fe²⁺ ions).
- After 1 hour, Cu²⁺ is replaced by Fe²⁺, changing the color to pale green.
Q9: The property by virtue of which a solid material can be drawn into thin wires is called:
(a) Malleability
(b) Ductility
(c) Rigidity
(d) Resistivity
 View Answer
View Answer 
Ans: (b) Ductility
- Ductility allows metals to be drawn into wires.
- Malleability is for hammering into sheets.
- Rigidity refers to stiffness, and resistivity is electrical resistance.
Q10: Which one of the following metals is protected from corrosion by a layer of its own oxide?
(a) Aluminium
(b) Copper
(c) Silver
(d) Gold
 View Answer
View Answer 
Ans: (a) Aluminium
- Aluminium forms a protective Al₂O₃ layer, preventing further corrosion.
- Copper forms a green patina (Cu₂(OH)₂CO₃), not just oxide.
- Silver and gold are resistant but do not rely on oxide layers.
Q11: Assertion (a): Hydrogen gas is not evolved when a metal reacts with nitric acid.
Reason (R): Nitric acid is a strong reducing agent and reduces the hydrogen produced in the reaction to water.
 View Answer
View Answer 
Ans: (c) Assertion (a) is true, but Reason (R) is false.
- A: Most metals do not produce H₂ with HNO₃ because it’s a strong oxidizing agent, oxidizing H₂ to H₂O.
- R: Nitric acid is an oxidizing agent, not a reducing agent.
- Thus, A is true, R is false.
Q12: Assertion (a): The metals high up in the reactivity series cannot be obtained from their compounds by heating with carbon.
Reason (R): Displacement reactions can also be used to obtain metal.
 View Answer
View Answer 
Ans: (b) Both Assertion (a) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (a).
- A: Highly reactive metals (e.g., Na, K, Al) have strong bonds in oxides, requiring electrolysis, not carbon reduction.
- R: Displacement reactions (e.g., thermite) are used for some metals, but this does not explain A.
Q13: Assertion (a): Ductility is that property of metals which enables copper to be used in making cooking utensils.
Reason (R): Copper is a metal which is ductile as well as malleable.
 View Answer
View Answer 
Ans: (d) Assertion (a) is false, but Reason (R) is true.
- A: Cooking utensils require malleability (shaping into sheets) and thermal conductivity, not ductility (wire drawing).
- R: Copper is both ductile and malleable, so R is true.
Q14: Assertion (a): Brass is prepared by first melting copper and then dissolving tin into it in a definite proportion.
Reason (R): The primary metal of brass is copper.
 View Answer
View Answer 
Ans: (c) Assertion (a) is true, but Reason (R) is false.
- A: Brass is made by melting copper and adding zinc (not tin).
- R: Copper is the primary metal in brass, but A mentions tin, making it incorrect. Corrected A would make R true, but as stated, R does not explain A.
Q15: Assertion (a): Silver chloride turns grey in sunlight.
Reason (R): Decomposition of silver chloride into silver and chlorine takes place by sunlight.
 View Answer
View Answer 
Ans: (a) Both Assertion (a) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (a).
- A: AgCl turns grey due to formation of silver metal.
- R: 2AgCl → 2Ag + Cl₂ (photolytic decomposition), explaining the grey color.
Q16: The main observations while performing the experiment of burning magnesium ribbon in air are:
(i) Magnesium ribbon burns with a dazzling white flame.
(ii) A white powder is formed.
(iii) Magnesium ribbon vapourises.
(iv) Aqueous solution of the white powder turns blue litmus to red.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
 View Answer
View Answer 
Ans: (c) (i) and (ii)
- (i) Mg burns with a dazzling white flame, correct.
- (ii) Forms white MgO powder, correct.
- (iii) Mg does not significantly vaporize; it reacts with O₂.
- (iv) MgO forms Mg(OH)₂ in water, which is basic and turns red litmus blue, not blue to red.
Q17: Select from the following a statement which is not true about burning of magnesium ribbon in air:
(a) It burns with a dazzling white flame.
(b) A white powder is formed on burning.
(c) It is an endothermic reaction.
(d) It is an example of a combination reaction.
 View Answer
View Answer 
Ans: (c) It is an endothermic reaction.
- 2Mg + O₂ → 2MgO is exothermic, releasing heat and light.
- (a), (b), and (d) are true: white flame, white MgO powder, combination reaction.
Q18: Study the following cases:
(i) CuSO₄ + Mg →
(ii) FeSO₄ + Pb →
(iii) CaSO₄ + Al →
(iv) ZnSO₄ + Ca →
The case/cases in which new product(s) will form is/are:
(a) Only (i)
(b) Only (iii)
(c) (i) and (iv)
(d) (i), (ii) and (iv)
 View Answer
View Answer 
Ans: (a) Only (i)
- Reactivity series: Mg > Al > Zn > Fe > Pb > Cu.
- (i) Mg displaces Cu: Mg + CuSO₄ → MgSO₄ + Cu.
- (ii) Pb is below Fe, no reaction.
- (iii) Al is below Ca, no reaction.
- (iv) Ca is below Zn, no reaction.
Q19: While burning a magnesium ribbon in air, list two safety measures which should be followed. Also state two observations of this activity.
 View Answer
View Answer 
Ans:
Safety Measures:
- Wear safety goggles to protect eyes from the dazzling flame.
- Use tongs to hold the ribbon to avoid burns.
Observations:
- Burns with a dazzling white flame.
- Forms a white powder (MgO).
Explanation:
- The bright flame can harm eyes, and the hot ribbon can cause burns.
- Reaction: 2Mg + O₂ → 2MgO, producing intense light and white ash.
Q20: (a) In common practice silver is recovered from silver nitrate solution by the use of copper metal. Name the type of reaction that takes place in this process and give the chemical equation of the reaction involved.
(b) Name the method used for refining silver.
 View Answer
View Answer 
Ans:
(a) Type: Displacement reaction.
Equation: Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag
(b) Method: Electrolytic refining.
Explanation:
- Cu, more reactive than Ag, displaces Ag from AgNO₃.
- Electrolytic refining purifies silver using a silver anode and cathode in an electrolyte.
Q21: Cinnabar is an ore of a metal 'X'. When this ore is heated in air, it is first converted into oxide of 'X' (XO) and then reduced to metal 'X' on further heating. Identify metal 'X' and write chemical equations for the reactions that occur in the above processes.
 View Answer
View Answer 
Ans:
Metal X: Mercury (Hg).
Equations:
- 2HgS + 3O₂ → 2HgO + 2SO₂
- 2HgO → 2Hg + O₂
Explanation:
- Cinnabar (HgS) is roasted to form mercury(II) oxide (HgO).
- HgO decomposes on heating to yield mercury metal.
Q22: Name a metal found in the earth's crust (i) in free state and (ii) in the form of its compound. State where each of these metals are placed in the reactivity series of metals.
 View Answer
View Answer 
Ans:
(i) Free State: Gold (Au), low in reactivity series (below Cu).
(ii) Compound: Aluminium (Al, as bauxite Al₂O₃), high in reactivity series (above Zn).
Explanation:
- Gold, being unreactive, exists native.
- Aluminium, reactive, forms stable compounds like oxides.
Q23: (a) Show the formation of magnesium chloride by electron transfer. Write the name of the cation and anion present in the compound formed. (Atomic Number of Mg=12, Cl=17)
OR
(b) How is zinc extracted from its ore? Name the processes involved in the extraction and write chemical equations for the reactions that occur during these processes.
 View Answer
View Answer 
Ans: (a)
Formation:
- Mg (2,8,2) → Mg²⁺ (2,8) + 2e⁻
- Cl (2,8,7) + e⁻ → Cl⁻ (2,8,8) [×2 for 2Cl]
- Mg + 2Cl → MgCl₂ (Mg²⁺[Cl⁻]₂)
Cation: Mg²⁺; Anion: Cl⁻
Explanation:
- Mg donates 2 electrons to two Cl atoms, forming ionic MgCl₂.
Q24: (a) Show the formation of calcium chloride by the transfer of electrons from one element to the other. Atomic Number of calcium and chlorine is 20 and 17 respectively.
OR
(b) "Aluminium oxide is an amphoteric oxide." Justify this statement giving chemical equation for the reactions involved.
 View Answer
View Answer 
Ans: (b)
Statement: Aluminium oxide (Al₂O₃) is amphoteric, reacting with both acids and bases.
Equations:
- With acid: Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O
- With base: Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O
Explanation:
- Al₂O₃ reacts with HCl to form a salt (like a base) and with NaOH to form sodium aluminate (like an acid), proving amphoteric nature.
Q25: (a) With the help of an activity, explain the conditions under which iron articles get rusted.
OR
(b) (i) Name two metals which react violently with cold water. List any three observations which a student notes when these metals are dropped in a beaker containing water.
(ii) Write a test to identify the gas evolved (if any) during the reaction of these metals with water.
 View Answer
View Answer 
Ans: (a)
Activity: Place three iron nails in test tubes:
- Tube A: Iron nail in water with air.
- Tube B: Iron nail in boiled water (no oxygen) with oil layer.
- Tube C: Iron nail in dry air (no moisture).
Observations:
- Tube A: Rust (Fe₂O₃·nH₂O) forms; requires water and oxygen.
- Tube B: No rust; oxygen absent.
- Tube C: No rust; moisture absent.
Conclusion: Iron rusts only with both water and oxygen.
Explanation:
- Rusting is a chemical reaction: 4Fe + 3O₂ + 2nH₂O → 2Fe₂O₃·nH₂O.
- Moisture and oxygen are essential for corrosion.
Q26: (a) "Displacement reactions also play a key role in extracting metals in the middle of the reactivity series." Justify this statement with two examples.
(b) Why can metals high up in the reactivity series not be obtained by reduction of their oxides by carbon?
 View Answer
View Answer 
Ans:
(a) Justification:
Displacement reactions reduce metal oxides using more reactive metals.
Examples:
- Fe₂O₃ + 2Al → 2Fe + Al₂O₃ (thermite, extracts iron).
- Cr₂O₃ + 2Al → 2Cr + Al₂O₃ (extracts chromium).
(b) Reason: Highly reactive metals (e.g., Na, K, Al) form very stable oxides due to strong metal-oxygen bonds, which carbon cannot break. Electrolysis is required.
Explanation:
- Middle-series metals (Fe, Zn) have less stable oxides, reducible by Al.
- High-series metals need stronger reducing conditions (electrolysis).
Q27: Write the electron-dot structures of (i) sodium, and (ii) oxygen. Using these structures, show the formation of sodium oxide. Mark the anion and cation present in this compound. (At. No. - Sodium = 11, Oxygen = 8)
 View Answer
View Answer 
Ans:
Electron-dot structures:
- Sodium (Na): 2,8,1 → Na·
- Oxygen (O): 2,6 → :Ö:
Formation of Na₂O:
- 2Na· → 2Na⁺ + 2e⁻
- :Ö: + 2e⁻ → [Ö]²⁻
- 2Na⁺ + [Ö]²⁻ → Na₂O
Cation: Na⁺; Anion: O²⁻
Each Na donates 1 electron to O, forming ionic Na₂O.
Q28: Design an activity to show that metals are good conductors of heat and have high melting points.
 View Answer
View Answer 
Ans:
Activity for Conductivity:
- Take a metal rod (e.g., copper) and fix thumbtacks with wax at equal intervals.
- Heat one end with a burner.
Observation: Wax melts sequentially from the heated end, showing heat conduction.
Activity for Melting Point:
Heat small pieces of copper and plastic in separate crucibles.
Observation: Plastic melts quickly; copper requires much higher heat, indicating a high melting point.
Explanation:
- Metals conduct heat due to free electrons.
- High melting points result from strong metallic bonds.
Q29: Samples of four metals A, B, C, and D were added one by one to the following solutions. The results obtained were tabulated as follows: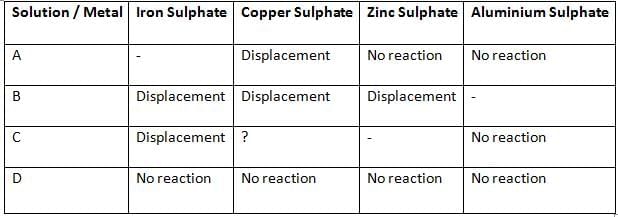
(i) Which is the least reactive metal?
(ii) What would be observed if C is added to a solution of copper sulphate?
(iii) Arrange the metals A, B, C, and D in the order of their decreasing reactivity.
 View Answer
View Answer 
Ans:
(i) Least Reactive: D
(ii) Observation: C displaces Cu from CuSO₄ (CuSO₄ + C → CSO₄ + Cu).
(iii) Order: B > C > A > D
Explanation:
- Reactivity series: More reactive metal displaces less reactive one.
- D: No reactions, least reactive.
- A: Displaces Cu, below Zn and Al.
- C: Displaces Fe, below Al, likely displaces Cu.
- B: Displaces Fe, Cu, Zn, most reactive.
Q30: Name and describe the most widely used method for refining impure metals?
 View Answer
View Answer 
Ans:
Method: Electrolytic refining.
Description:
- Impure metal is the anode, pure metal is the cathode.
- Electrolyte is a solution of the metal’s salt (e.g., CuSO₄ for copper).
- On passing current, metal ions from the anode dissolve, deposit as pure metal on the cathode.
- Impurities (sludge) settle at the bottom.
Explanation:
Used for metals like Cu, Al, ensuring high purity.
Q31: (a) Observe the following diagram showing an experiment to determine the conditions under which a metal 'M' corrodes. List your observations in each of the three cases A, B, and C with reason, if the metal 'M' is generally protected against corrosion by the method of galvanisation.
OR
(b) (a) Show the formation of Aluminium Nitride (AlN) by the transfer of electrons. [At. no. of Al = 13; N = 7]
(b) "Ionic compounds are solids and are generally brittle and break into pieces when pressure is applied." Give reason to justify the statement.
 View Answer
View Answer 
Ans: (b)
(a) Formation of AlN:
- Al (2,8,3) → Al³⁺ (2,8) + 3e⁻
- N (2,5) + 3e⁻ → N³⁻ (2,8)
- Al³⁺ + N³⁻ → AlN
(b) Reason: Ionic compounds have strong electrostatic forces forming a rigid lattice, but pressure displaces ions, causing like charges to repel, leading to brittleness.
Explanation:
- Al donates 3 electrons to N, forming ionic AlN.
- Ionic lattices break when lattice alignment is disrupted.
Q32: (a) What is a reactivity series of elements? How is it developed? Arrange the following elements as they are arranged in the reactivity series: Aluminum, Calcium, Copper, Lead
(b) Write balanced chemical equation to show the reaction of iron (III) oxide (Fe₂O₃) with aluminium.
 View Answer
View Answer 
Ans:
(a) Reactivity Series: A list of metals arranged in order of decreasing reactivity.
Development: Based on displacement reactions and tendency to lose electrons.
Order: Ca > Al > Pb > Cu
(b) Equation: Fe₂O₃ + 2Al → 2Fe + Al₂O₃
Explanation:
- Reactivity is tested by observing which metals displace others from solutions.
- Thermite reaction reduces Fe₂O₃ to iron.
Q33: (a) (i) Consider the following metals: K, Ca, Al, Cu, Ag, Fe
Select from the above metals, a metal which:
I. Does not react with oxygen even at high temperature.
II. Reacts with oxygen at ordinary temperature and forms a protective oxide layer which prevents the metal from further oxidation.
III. Catches fire when kept in the open.
IV. Does not burn in oxygen but the hot metal is coated with a black coloured oxide layer.
(ii) What are amphoteric oxides? With the help of balanced chemical equations show that aluminium oxide is an amphoteric oxide.
(iii) What are alkalis? Give one example.
 View Answer
View Answer 
Ans: (a) (i)
- I: Ag (silver, unreactive).
- II: Al (forms protective Al₂O₃).
- III: K (catches fire spontaneously).
- IV: Cu (forms black CuO on heating).
(ii) Amphoteric Oxides: React with both acids and bases.
- Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O
- Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O
(iii) Alkalis: Water-soluble bases. Example: NaOH.
Explanation:
- Al₂O₃ shows dual reactivity, confirming amphoteric nature.
- Alkalis dissolve in water, producing OH⁻ ions.
Q34: Many pure metals like copper, iron, and gold are very soft and as such are considered unsuitable for certain uses. Metallic objects around us such as cooking utensils, statues, ornaments, guns, etc., are actually not made up of pure metals. Instead of pure metals, alloys are used in the design of most of the useful objects. Making alloys enhances the basic properties of a metal which is the primary constituent (metal) of an alloy.
(I) How does electrical conductivity and melting point of a metal change when it is converted to its alloy by mixing a small amount of an element in it?
(II) Name an alloy used for welding two wires together in an electric circuit. Write its major constituents.
(III)(a) What are alloys? How is 'Brass' (an alloy) prepared?
 View Answer
View Answer 
Ans:
(I) Changes:
- Electrical conductivity decreases due to disruption of metallic lattice by alloying element.
- Melting point usually decreases as alloys have less ordered structures.
(II) Alloy: Solder; Constituents: Lead, Tin.
(III)(a) Alloys: Mixtures of two or more metals (or metal and non-metal) to enhance properties. - Brass Preparation: Melt copper, add zinc in specific proportions, and cool.
Explanation:
- Alloys improve strength and corrosion resistance.
- Solder’s low melting point aids welding.
- Brass (Cu + Zn) is harder than pure copper.
FAQs on Previous Year Questions: Metals and Non-metals - Class 10
| 1. What are the main differences between metals and non-metals? |  |
| 2. Why are metals good conductors of electricity? |  |
| 3. What are some common properties of non-metals? |  |
| 4. How do metals and non-metals react during chemical reactions? |  |
| 5. What are some uses of metals and non-metals in everyday life? |  |













