The Amazing World of Solutes, Solvents, and Solutions Chapter Notes | Chapter Notes For Class 8 PDF Download
Have you ever wondered why sugar completely disappears when you stir it in water, but sand does not?
Welcome to The Amazing World of Solutes, Solvents, and Solutions! In this chapter, you’ll explore what happens at the microscopic level when different substances mix, why some mixtures turn clear while others stay cloudy, and how countless solutions around us—from tasty juices to salty seas—are a big part of daily life. Let’s dive in and discover the science behind the solutions you encounter every day!
What Are Solute, Solvent, and Solution?
What Is a Solution?
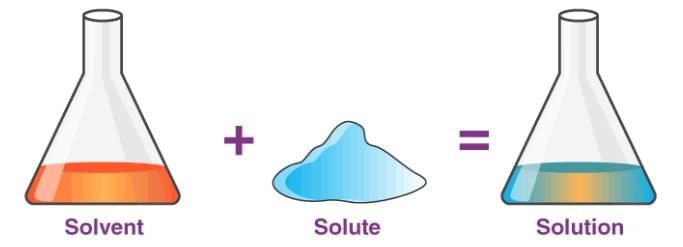
- A solution is a uniform (homogeneous) mixture where components are evenly distributed and cannot be seen separately.
- Example: Mixing salt or sugar with water forms a clear solution
- Sand or sawdust in water settles or separates, not forming a solution
- Basic Formula: Solute + Solvent = Solution
Key Definitions
- Solute: The substance that dissolves in the solvent to form a solution. It is usually the component present in a smaller amount. In solid-liquid solutions: The solid (e.g., salt or sugar) is the solute.
- Solvent: The substance that dissolves the solute. It is usually the component present in a larger amount and determines the state of the solution. In solid-liquid solutions: The liquid (e.g., water) is the solvent.
Solution Formation: The solute mixes completely with the solvent, creating a uniform mixture where particles are too small to see or separate easily.
Types of Solutions and Examples
Solid in Liquid:
Solute: Solid (e.g., salt, sugar).
Solvent: Liquid (e.g., water).
Example: Saltwater or sugar water—uniform and clear.
Liquid in Liquid:
Not always clear which dissolves which.
Rule: Substance in smaller amount = solute; larger amount = solvent.
Example: Vinegar (acetic acid in water)—acetic acid (smaller) is solute, water (larger) is solvent.
Gas in Gas (Gaseous Solutions):
- Gases can form solutions too. Example:Air is a gaseous solution.
- Solvent: Nitrogen (largest amount, about 78%).
- Solutes: Oxygen, argon, carbon dioxide, and other gases (smaller amounts).
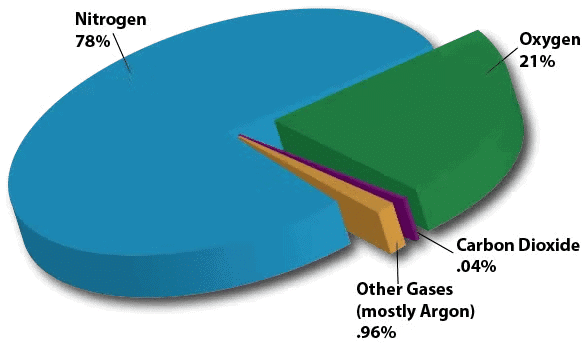 Air is a mixture of Gases
Air is a mixture of Gases
Special Cases and Interesting Facts
- When Solute is More Than Solvent: Even if the solute amount is larger, the liquid is still the solvent.
In Chashni (sugar syrup for Gulab Jamun), a large amount of sugar (solid solute) dissolves in a small amount of water (liquid solvent). Water remains the solvent despite being less.
How Much Solute Can a Fixed Amount of Solvent Dissolve?
Capacity of Water to Dissolve Solutes
The experiment/steps below demonstrate the limits of dissolution by gradually adding salt to water and observing when it stops dissolving. It illustrates that solvents have a maximum capacity for solutes, leading to concepts of saturation and solubility.
- Take a clean glass tumbler half-filled with water.
- Add one spoonful of salt and stir until it dissolves completely.
- Continue adding spoonfuls of salt, stirring each time, and observe dissolution.
Observations and Inferences:
- Initially, salt dissolves completely, forming a clear solution.
- After a few spoons (varies, but typically 3–5 depending on water volume), added salt does not dissolve and settles at the bottom.
- This indicates water reaches a limit and cannot dissolve more salt.
Concepts:
- Typically 3–4 spoons before undissolved salt remains (exact number depends on water amount and temperature).
- Water has a finite ability to dissolve salt; adding more beyond this limit results in undissolved solute settling.
- If more salt is added to a given amount of water, it remains undissolved, showing the solvent's dissolution limit.
Important Definitions:
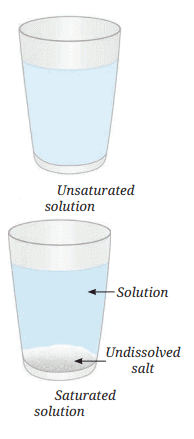
Unsaturated Solution: A solution where more solute can be dissolved at a given temperature (e.g., initial stages with 1–2 spoons of salt).
Saturated Solution: A solution where no more solute can dissolve at a particular temperature, and excess solute settles at the bottom.

Concentration: The amount of solute present in a fixed quantity of solution (or solvent); it determines how "strong" the solution is.
Dilute Solution: A solution with a relatively small amount of solute in the solvent (e.g., 1 spoon of salt in water is dilute compared to 2+ spoons).
Concentrated Solution: A solution with a relatively large amount of solute in the solvent (e.g., more spoons of salt make it more concentrated).
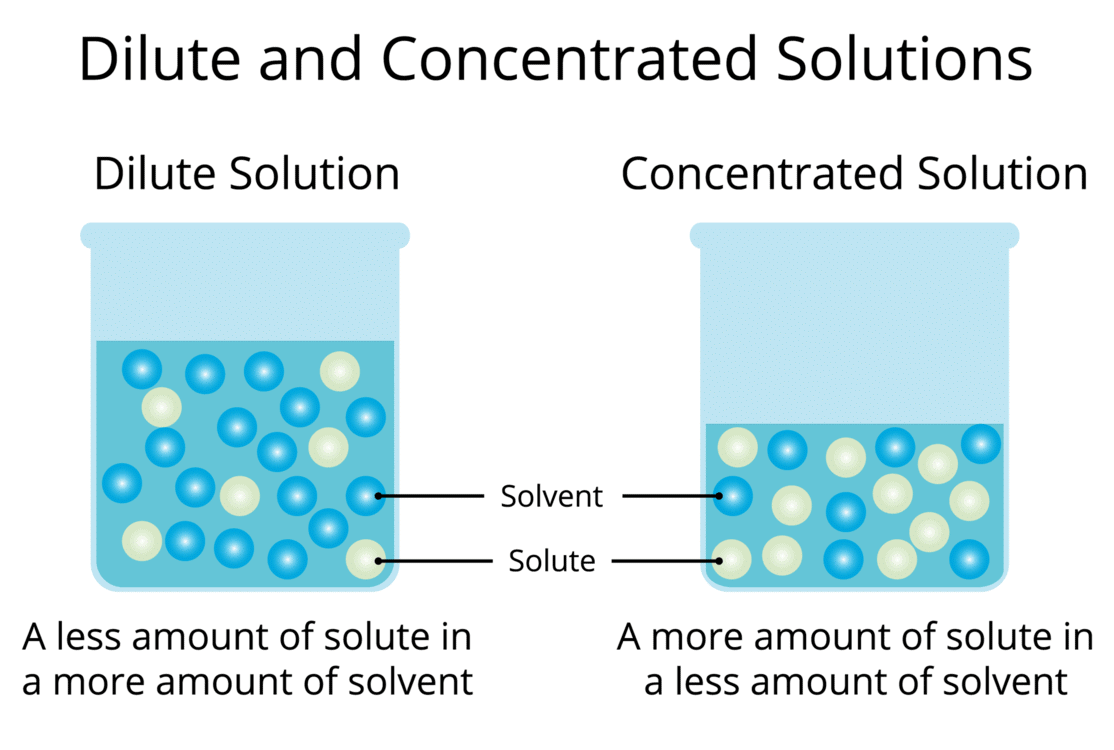
Relative Terms: Dilute and concentrated are comparative; for example, 2 spoons of salt in 100 mL water is less concentrated than 4 spoons in 50 mL water.
Solubility: The maximum amount of solute that can dissolve in a fixed quantity of solvent at a given temperature; it represents the solvent's dissolving capacity.
Effect of Temperature on Solubility
The following experiment/ steps show how heating affects a solute's dissolution, which shows that solubility generally increases with temperature.
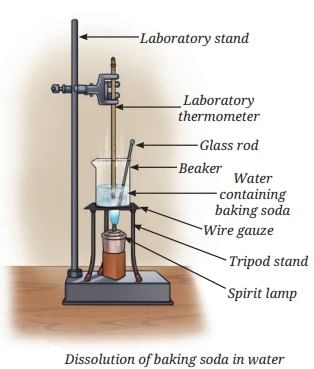
Steps:
- Take 50 mL of water in a glass beaker and measure its temperature (e.g., 20°C) using a laboratory thermometer.
- Add a spoonful of baking soda (sodium hydrogen carbonate) and stir until it dissolves; continue adding small amounts while stirring until some remains undissolved at the bottom.
- Heat the mixture to 50°C while stirring.
- Observe the undissolved baking soda dissolving.
- Add more baking soda until undissolved solid remains again.
- Heat further to 70°C while stirring and observe again.
Observations and Inferences:
- At 20°C: Limited baking soda dissolves; excess remains undissolved.
- At 50°C: Previously undissolved baking soda dissolves; more can be added before saturation.
- At 70°C: Even more baking soda dissolves, showing increased capacity.
- Inference: Water at 70°C dissolves more baking soda than at 50°C, and much more than at 20°C.
Important Definitions:
Temperature Effect on Solubility: For most substances, solubility increases with rising temperature (e.g., a saturated solution at lower temperature becomes unsaturated when heated, allowing more solute to dissolve).
Practical Implication: Heating can turn a saturated solution into an unsaturated one, enabling more dissolution.
Our Scientific Heritage: Solvents in Traditional Indian Medicine
Water is primarily used as a solvent for preparing medicinal formulations in systems like Ayurveda, Siddha, and other traditional Indian medicines.
Hydro-alcoholic extracts: Herbs are mixed with water-alcohol solutions to create drug formulations.
Other solvents mentioned: Oils, ghee, milk, and similar substances are used to enhance therapeutic benefits of drugs.
Be a Scientist: Asima Chatterjee's Contributions
Inspiration: Asima Chatterjee was inspired to work on medicinal plants, focusing on extracting and isolating important compounds using solvents and solutions.
Asima Chatterjee
Achievements:
Renowned for developing anti-epileptic and anti-malarial drugs from plants.
Earned a Doctorate of Science (second Indian woman after Janaki Ammal).
First woman to receive the Shanti Swarup Bhatnagar Award in chemical science.
Honored with the Padma Bhushan for her contributions.
Solubility of Gases
- Many gases, including oxygen, can dissolve in water.
- Oxygen dissolves only to a small extent (minute quantities), but this is crucial for life.
- Importance for Aquatic Life: The dissolved oxygen sustains all aquatic organisms, such as plants, fishes, and other life forms—even in small amounts.

Nature of Gas-Liquid Mixtures
- Uniform or Non-Uniform?: The mixture of gases (like oxygen) in water is a uniform mixture (homogeneous).
- Reason: Gases dissolve evenly throughout the water, forming a solution where no separate layers or particles are visible.
Effect of Temperature on Gas Solubility
- Does Temperature Affect It?: Yes, temperature impacts the solubility of gases in liquids.
- How It Affects: Solubility of gases generally decreases as temperature increases.
- Examples:
Cold Water: More oxygen can dissolve, providing sufficient oxygen for aquatic life.
Warm Water: Solubility of oxygen decreases, resulting in less dissolved oxygen. Key Inference: Unlike solids (where solubility often increases with temperature), gases behave oppositely—higher temperatures make it harder for them to stay dissolved.
Why Do Objects Float or Sink in Water?
- You must have observed that some objects float while others sink in water
- You may have noticed that, while washing rice, husk particles present in the rice float on the surface of water while rice sinks to the bottom of the container. Question: Why does this happen?
- Another Example: If you add oil to water, it floats on water.
- Generally, it is believed that objects that float in a liquid are lighter and others that sink are heavier than the liquid.
- A wooden stick and an iron rod may be of the same size, yet the iron rod feels much heavier.
- Explanation of Heaviness: When we say that iron is heavier than wood, we are referring to a special property known as density, which describes the heaviness of an object.
- Note: However, the density of a substance is not the only factor that decides whether it will float or sink in a particular liquid.
What is Density?
- Imagine a crowded bus where many people are packed together—this is an example of high density. The same bus with only a few people is an example of low density.
- Similarly, a forest where trees grow close to each other is called a dense forest, but if the trees are far apart, it is considered less dense.
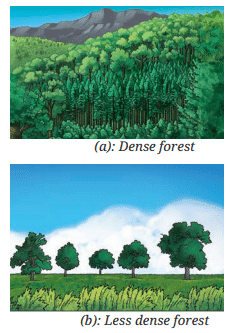
- Question: How do scientists define density? Let us find out.
- Recall: We have learnt that matter is anything that possesses mass and occupies space (volume).
Definition and Formula of Density
- Density Definition: Density is defined as the mass present in a unit volume of that substance.
- Mathematical Formula: Density = Mass / Volume.
- The density of a substance is independent of its shape or size.
- However, it is dependent on temperature and pressure.
- Pressure primarily affects the density of gases, while effect of pressure on the density of solids and liquids is negligible.
Oil Packet Example
- Have you noticed that some packets of ghee or oil are labelled with a volume of 1 litre but a weight of only say 910 grams.
- What This Tells Us: The density of the oil is less than that of water.
- A 1-litre packet of oil weighing 910 grams indicates a density of 0.91 g/cm³ (since 1 litre = 1000 cm³, and 910 g ÷ 1000 cm³ = 0.91 g/cm³). Water has a density of 1 g/cm³. Since 0.91 g/cm³ is less than 1 g/cm³, the oil is less dense than water.
Units of Density
The units in which density is expressed will depend upon the units of mass and volume taken.
SI Units: The SI units of mass and volume are kilogram (kg) and cubic metre (m³), respectively. Therefore, the SI unit of density is kilogram per cubic metre, abbreviated as kg/m³.
Units for Liquids: In case of liquids, other units of density are also used for convenience, such as gram per millilitre, abbreviated as g/mL and gram per cubic centimetre, abbreviated as g/cm³.
Conversion Factor for Density
1 kg/m³ = 1000 g/m³ = 1000 g/1000 L = 1 g/L = 1 g/1000 mL = 1 g/1000 cm³- The mass of 1 mL of water is close to 1 g at room temperature.
- For the measurement of the mass of water, we generally consider the volume in mL and its mass in g.
- Hence, 10 mL of water would be approximately 10 g. Similarly, 100 mL of water would be approximately 100 g.
Relative Density Example and Definition
- Suppose the mass of an aluminium block is 27 g and its volume is 10 cm³, its density is 2.7 g/cm³.
- From this, it can be said that aluminium is 2.7 times denser than water.
- We express this fact by saying that the relative density of aluminium with respect to water is 2.7. It is a number without any units.
- Formula for Relative Density: Relative density of any substance with respect to water = Density of that substance / Density of water at that temperature.
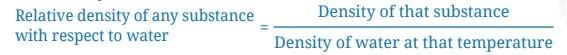
Determination of Density
The density of an object can be determined by measuring its mass and volume.
How to Measure Mass?
- Mass Definition: Mass is the quantity of matter present in any object.
- Instrument: The instrument used to measure the mass of an object is known as a balance.
- Concept of Using a Digital Balance: A digital weighing balance shows mass readings directly.
- Zero Reading Concept: The balance should show a zero reading initially. If not, press the tare or reset button to set it to zero.
- Taring Concept: Place a dry and clean watch glass or butter paper on the pan, note the reading, then reset to zero using the tare button. This ignores the container's mass.
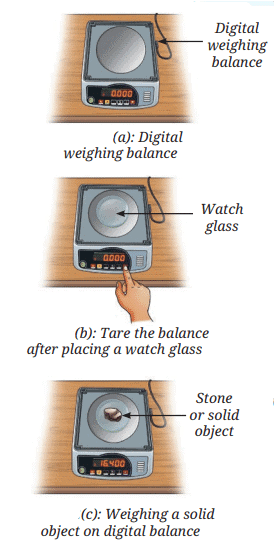
- Measuring Solid Objects: Carefully place the solid object (e.g., a stone) on the watch glass and note the reading, which gives the mass. You may use any other type of balance available in your school.
- Note on Liquids: The mass of a liquid may be measured by replacing the watch glass with a beaker and pouring the desired amount of liquid into it.
Mass vs. Weight
- The words ‘mass’ and ‘weight’ are often used interchangeably in everyday language. But they have different meanings in science, which can sometimes cause confusion.
- Mass: Is the quantity of matter present in an object or a substance. Its units are gram (g) and kilogram (kg).
- Weight: Is the force by which the Earth attracts an object or a substance towards itself, and it is measured in newtons (N).
- Most balances actually measure weight, but their scales are marked in mass units, so they show values in grams or kilograms.
How to Measure Volume?
- Volume is the space occupied by an object.
- You also know that the SI unit of volume is cubic metres, written as m³. It is the volume of a cube whose each side is one metre in length.
- Volume of smaller objects is conveniently expressed in a decimetre cube (dm³) or centimetre cube (cm³). One centimetre cube is also written as one cc.
- Volume of liquids is expressed in litres (L) which is equivalent to 1 dm³.
- A commonly used submultiple of a litre is millilitre (mL) which is equivalent to 1 cm³.
- One of the common apparatuses used to measure the volume of liquids is a measuring cylinder. It is a narrow transparent cylindrical container with one side open and the other side closed.
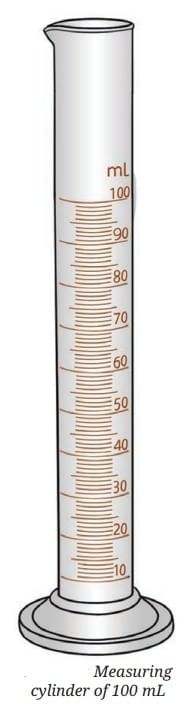
- There are markings on the transparent body of the cylinder that indicate the volume of liquid in the measuring cylinder.
- We can use it to measure the desired amount of a liquid.
- Measuring cylinders are available in different sizes to measure volume—5 mL, 10 mL, 25 mL, 50 mL, 100 mL, 250 mL, etc.
Observing and Calculating with Measuring Cylinder
- Concept of Capacity: The measuring cylinder can measure up to its marked maximum volume (e.g., 100 mL).
- Smallest Volume Concept: The smallest volume it can measure is found by checking divisions.
- Division Calculation: Volume difference between bigger marks (e.g., 10 mL to 20 mL) is 10 mL. If there are 10 smaller divisions, each is 1 mL (10 ÷ 10 = 1 mL).
- Accuracy Depends on Size: Smaller cylinders (e.g., 10 mL or 25 mL) measure 0.1 mL accurately.
- Choosing the Right Cylinder: For 70 mL, a 100 mL cylinder is best (accurate, one step). 50 mL needs two steps (less convenient). Larger ones (250 mL or 500 mL) are less accurate for small amounts.
Key Concepts: Measuring 50 mL of Water
- Setup Concept: Place a clean, dry measuring cylinder on a flat surface and pour water slowly to the mark.
- Adjustment Concept: Use a dropper to add or remove water for exact level.
- Meniscus Concept: Water forms a curved surface called the meniscus. Read the bottom of the meniscus for water or colorless liquids.
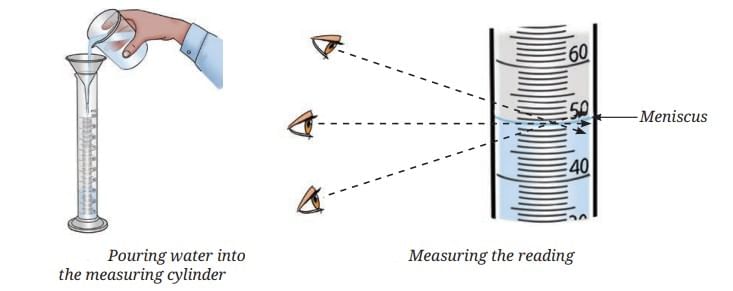
- Reading Concept: Keep eyes at level with the bottom of the meniscus for accurate reading.
- Transfer Concept: Once at 50 mL, pour into the required container.
- For Colored Liquids: Read the top of the meniscus.
Determining Volume of Solid Objects with Regular Shapes
- Concept: For cuboid shapes (e.g., notebook, shoe box, dice), measure length (l), width (w), height (h) with a scale.
- Formula: Volume = l × w × h.
- Example: Notebook: l = 25 cm, w = 18 cm, h = 2 cm → Volume = 25 × 18 × 2 = 900 cm³.
Determining Volume of Objects with Irregular Shapes
For irregular objects like a stone, volume is hard to measure directly. Use water displacement in a measuring cylinder.
Fill cylinder with water (e.g., 50 mL initial;) and record.
Tie object (e.g., stone) with thread and lower slowly into water.
Water level rises (e.g., to 55 mL)—this is displacement.
Volume of object = Final volume - Initial volume (e.g., 5 mL).
Units: mL for liquids = cm³ for solids.
Calculating Density
Use formula: Density = Mass / Volume.
Question: An object has a mass of 16.400 g and a volume of 5 cm³. Calculate its density using the formula Density = Mass / Volume. (Show your working and express the answer in g/cm³.)
Solution:
Density = 16.400 g / 5 cm³ = 3.28 g/cm³.
Did you know?
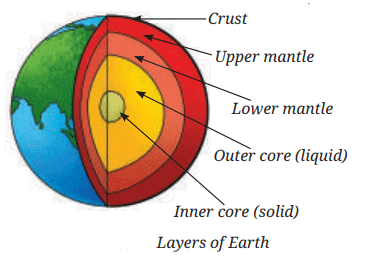
- Our planet, Earth, is composed of several layers, such as crust, upper mantle, lower mantle, outer core, and inner core, each with its particular range of density.
- The outermost layer, called the crust, is the lightest and the density of the different layers increases as we move towards the centre.
- As one moves deeper into the Earth, both the pressure and the temperature rise significantly, making the materials heavier and more compact.
Bamboo and Wooden Logs
- In ancient times, before large ships were invented, people used bamboo and wooden logs to travel across rivers and seas.
- Bamboo was used because it is light, hollow, and floats easily on water.

- People tied bamboo poles together to make rafts and small boats for fishing, trading, and crossing water bodies.
- Wooden logs, especially from strong trees were either hollowed out to make boats or used as rafts.
- These simple boats, made from locally available materials, were important for moving around and connecting different places.
- Even today, similar traditional boats made of bamboo or wood are used in some regions—not just for transport, but also as tourist attractions.
Effect of Temperature on Density
Generally, the density of a substance decreases with heating and increases with cooling.
- As temperature increases, the particles of a substance whether, solid, liquid, or gas, tend to move away and spread.
- This results in an increase in volume but there is no change in mass.
- Since the Density = Mass/Volume, upon heating mass remains same, the volume increases and the density decreases.
- This explains why hot air moves up as it is less dense than the cool air around it.

- The hot air balloon works on the same principle.
Effect of Pressure on Density
Pressure affects density differently depending on the state of matter.
- Gases: For gases, increasing pressure causes the particles to move closer together. As a result, the volume of the gas decreases and its density increases.
- Liquids: In the case of liquids, pressure has a small effect because they are nearly incompressible.
- Solids: The particles in solids are very close to each other. Solids are even less affected by pressure than liquids, and changes in their density are usually negligible.
Why Does Ice Float on Water?
- Ice floats on water because it is lighter than liquid water.

- Water has a special property that its density is highest at 4 °C. It means water is heaviest at 4 °C.
- As the temperature drops, and water turns into ice at 0 °C, it undergoes a change in structure—the particles arrange themselves in a way that takes up more space.
- This process is called expansion.
- Because the same amount of water now occupies a larger volume, its density decreases.
- As a result, ice becomes lighter than liquid water and floats on its surface.
- This is important for animals living in lakes and oceans because ice floats, it forms a layer on top, keeping the water underneath warm enough for fish and other creatures to survive, even in extremely cold weather.
Egg Experiment
Take a glass tumbler and fill it with tap water. Carefully place a raw whole egg into the water and observe what happens. You will notice that the egg sinks to the bottom.
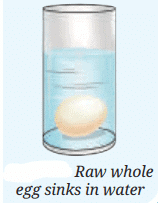
Key Terms to Remember
- Solution: A uniform mixture formed when two or more substances mix evenly together.
- Solute (in Solid-Liquid Solution): The solid component that dissolves in a liquid to form a solution.
- Solvent (in Solid-Liquid Solution): The liquid component in which a solid dissolves to form a solution.
- Solute (in Liquid-Liquid Solution): The component present in lesser quantity when two liquids mix to form a solution.
- Solvent (in Liquid-Liquid Solution): The component present in greater quantity when two liquids mix to form a solution.
- Solvent and Solutes in Air: In air, nitrogen acts as the solvent (major component), while oxygen, argon, carbon dioxide, and other gases are the solutes (minor components).
- Saturated Solution: A solution where the maximum amount of solute is dissolved, and no more can be added at that temperature.
- Unsaturated Solution: A solution where additional solute can still be dissolved at a given temperature.
- Solubility: The maximum amount of solute that can dissolve in a fixed quantity (100 mL) of solution or solvent at a specific temperature.
- Temperature Effect on Solubility: In liquids, the solubility of solids generally increases with rising temperature, while the solubility of gases decreases.
- Mass: The amount of matter present in an object.
- Volume: The space occupied by an object or substance.
- Devices for Measurement: A weighing balance measures mass, and a measuring cylinder measures volume.
- Density: The mass per unit volume of a substance, calculated as Density = Mass / Volume.
- Temperature and Pressure Effects on Density: Density generally decreases with increasing temperature; pressure affects density differently by state of matter (e.g., increases gas density by compressing volume).
FAQs on The Amazing World of Solutes, Solvents, and Solutions Chapter Notes - Chapter Notes For Class 8
| 1. What is the definition of a solute? |  |
| 2. What is the role of a solvent in a solution? |  |
| 3. How are solutions classified? |  |
| 4. What is an example of a solution in everyday life? |  |
| 5. How does temperature affect the solubility of a solute? |  |