Particulate Nature of Matter NCERT Solutions | Science Curiosity Class 8 - New NCERT PDF Download
| Table of contents |

|
| Probe and Ponder (Page 98) |

|
| InText (Page 99 - 110) |

|
| Keep the Curiosity Alive (Page 113 - 114) |

|
| Discover, Design, and Debate (Page 115) |

|
Probe and Ponder (Page 98)
Q1: Why is it possible to pile up stones or sand, but not a liquid like water?
Ans: Stones and sand are solids, where particles are tightly packed with strong interparticle attractions, giving them a fixed shape and allowing them to be piled up without flowing. Water is a liquid, with weaker interparticle attractions, so its particles can move freely and flow, taking the shape of the container or spreading out, making piling impossible.
Q2: Why does water take the shape of folded hands but lose that shape when released?
Ans: Water is a liquid, so its particles have enough freedom to move and adapt to the shape of the container (like folded hands). When released, the particles flow due to gravity and weaker interparticle forces, losing the shape because liquids do not have a fixed form—they only have a fixed volume.
Q3: We cannot see air, so how does it add weight to an inflated balloon?
Ans: Air is a gas made of tiny, invisible particles (like nitrogen and oxygen molecules) that are far apart and in constant motion. When a balloon is inflated, these particles occupy space inside, adding mass (and thus weight) to the balloon, even though the particles themselves are too small to see.
Q4: Is the air we breathe today the same that existed thousands of years ago?
Ans: Yes, in terms of composition, the air we breathe is largely the same mixture of gases (nitrogen, oxygen, etc.) that has existed for thousands of years, as matter is conserved and particles cycle through natural processes like respiration and photosynthesis. However, human activities have slightly altered its composition, adding pollutants.
Q5: Share your questions?
Ans:
- How small are the tiniest particles of matter, and can we ever see them?
- Why do some solids melt easily while others need very high temperatures?
- If gases have no fixed volume, how do they stay contained in the atmosphere?
- What happens to the particles when a substance changes from solid to liquid?
- Why don't all solids dissolve in water like sugar does?
InText (Page 99 - 110)
Q1: Is every speck of this fine chalk powder still composed of the same substance, or has it changed into something else on breaking or grinding? (Page 99)
Ans: Yes, even after breaking or grinding, each speck of chalk powder (fine-grinded) is the same as the previous state because this change is a physical change in which only the size of chalk changes, not any chemical change occurred.
Q2: Are the units of chalk obtained in this manner considered the smallest units of chalk? (Page 100)
Ans: No, the obtained units of chalk in the process of grinding are not the smallest unit. Every unit of chalk is even consists of constituent particles, which are the basic units of chalk.
Q3: Chalk and sugar can both be broken down into their constituent particles. But how are the constituent particles held together to form the solid pieces we see? (Page 101)
Ans: The constituent particles in solids, like chalk and sugar, are held together by strong forces of attraction. These forces keep the particles tightly packed, giving the solid its definite shape and size.
Q4: In the solid state, is there any way to move these particles apart? (Page 102)
Ans: In solids, the particles are very close to each other and can only vibrate in their fixed positions. They cannot move freely apart unless the solid is heated enough to melt into a liquid. Solid Particles
Solid Particles
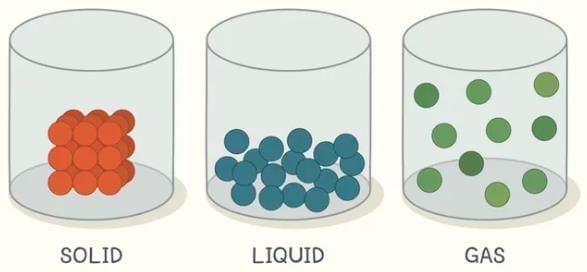
Q5: Solids have a definite volume; what about liquids and gases? (Page 103)
Ans:
- Liquids have a definite volume but no definite shape; they take the shape of the container.
- Gases have neither definite shape nor definite volume; they expand to fill the entire container.
Q6: Do gases also have a fixed volume? (Page 105)
Ans: No, gases don’t have a fixed shape or volume. The volume of gas changes with the amount of closeness of particles or the interparticle attraction between particles.
Q7: Sugar and sand are both solids. Why does sugar dissolve in water, but sand does not? (Page 108)
Ans: Sugar particles are solid, but they dissolve in water and occupy some space between the water molecules. Because water can break down sugar particles, which reduces the total volume of the mixture. Whereas sand particles have a rigid crystal structure, which cannot be broken down by water molecules, and hence settle down in water and increasing the total volume.
Q8: How can we demonstrate the movement of gas particles that cannot be seen with the naked eye? (Page 110)
Ans: We can show gas particle movement using smoke or colored gases. For example, smoke from incense spreads in air, showing that gas particles move randomly.
Keep the Curiosity Alive (Page 113 - 114)
Q1: Choose the correct option.
The primary difference between solids and liquids is that the constituent particles are:
(i) closely packed in solids, while they are stationary in liquids.
(ii) far apart in solids and have fixed position in liquids.
(iii) always moving in solids and have fixed position in liquids.
(iv) closely packed in solids and move past each other in liquids.
Ans: (iv) closely packed in solids and move past each other in liquids.
In solids, particles are fixed in position due to strong attractions, while in liquids, particles can slide past one another, allowing flow.
Q2: Which of the following statements are true? Correct the false statements.
(i) Melting ice into water is an example of the transformation of a solid into a liquid.
Ans: True
(ii) Melting process involves a decrease in interparticle attractions during the transformation.
Ans: False
The Melting process involves a decrease in interparticle attraction during the transformation.
(iii) Solids have a fixed shape and a fixed volume.
Ans: True
(iv) The interparticle interactions in solids are very strong, and the interparticle spaces are very small.
Ans: True
(v) When we heat camphor in one corner of a room, the fragrance reaches all corners of the room.
Ans: True
(vi) On heating, we are adding energy to the camphor, and the energy is released as a smell.
Ans: False
On heating, energy is added to camphor, causing it to undergo sublimation. The camphor directly converts into gas, and the vapour carries its characteristic smell.
Q3: Choose the correct answer with justification. If we could remove all the constituent particles from a chair, what would happen?
(i) Nothing will change.
(ii) The chair will weigh less due to lost particles.
(iii) Nothing of the chair will remain.
Ans: Correct option is (iii) Nothing of the chair will remain.
A chair is made up of constituent particles (atoms and molecules). If you remove all the particles from the chair, there is nothing left to form the structure, shape, weight, or existence of the chair.
Q4: Why do gases mix easily, while solids do not?
Ans: Gas particles are far apart from each other, and that’s why they move very fast in all directions. Gases have weak intermolecular forces, so they don’t attach. Due to this reason, gas particles spread easily around other particles.
Q5: When spilled on the table, milk in a glass tumbler flows and spreads out, but the glass tumbler stays in the same shape. Justify this statement.
Ans: In this case, milk is spilled on the table, and it spreads around the table because its state is liquid. Liquids can take the shape of their surrounding because their molecules are free to move. This is the reason the milk flows around the table. Whereas the glass tumbler’s shape does not change because it is a solid. In solids, the molecules are closely packed.
Q6: Represent diagrammatically the changes in the arrangement of particles as ice melts and transforms into water vapour.
Ans: As ice melts into water and then vaporizes into steam, the arrangement of water particles changes significantly. Initially, in ice (a solid), water molecules are tightly packed in a fixed, crystalline structure with limited movement (vibrations). As ice melts, the particles gain kinetic energy, breaking free from their fixed positions and becoming able to slide past each other, forming liquid water. Further heating increases the kinetic energy, causing the particles to move more rapidly and spread out, eventually breaking free from the liquid and becoming water vapour, a gas with particles moving randomly and freely.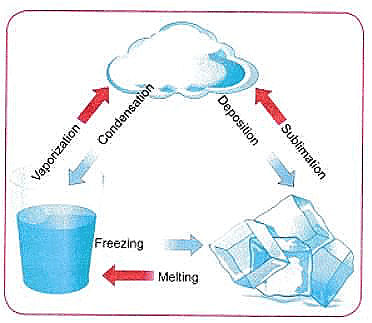
Ice (Solid)
- Arrangement: Water molecules are tightly packed in a regular, crystalline structure.
- Movement: Molecules vibrate in fixed positions.
Liquid Water
- Arrangement: Molecules are closer together than in a gas, but not in a regular structure. They can move around and slide past each other.
- Movement: Molecules can move around and slide past each other.
Water Vapor (Gas)
- Arrangement: Molecules are far apart and move randomly and freely in all directions.
- Movement: Molecules move rapidly and randomly, colliding with each other and the container walls.
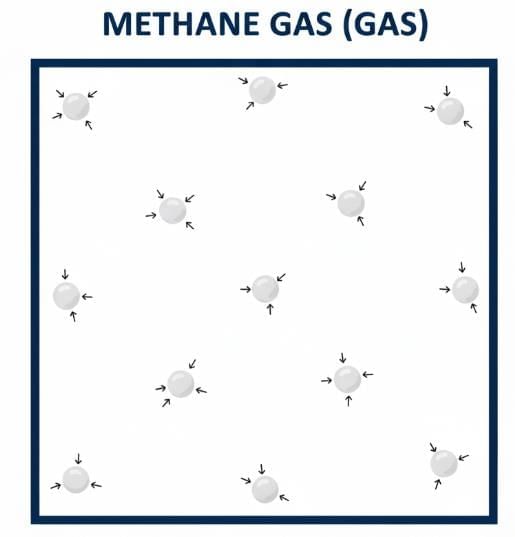
Q7: Draw a picture representing particles present in the following:
(i) Aluminium foil
(ii) Glycerin
(iii) Methane gas
Ans: Pictorial representation of particles of Aluminium foil, Glycerin, and Methane gas.

Q8: Observe Fig. 7.16a which shows the image of a candle that was just extinguished after burning for some time. Identify the different states of wax in the figure and match them with Fig. 7.16b showing the arrangement of particles.
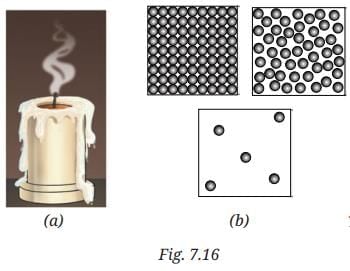 Ans: Different states of wax:
Ans: Different states of wax: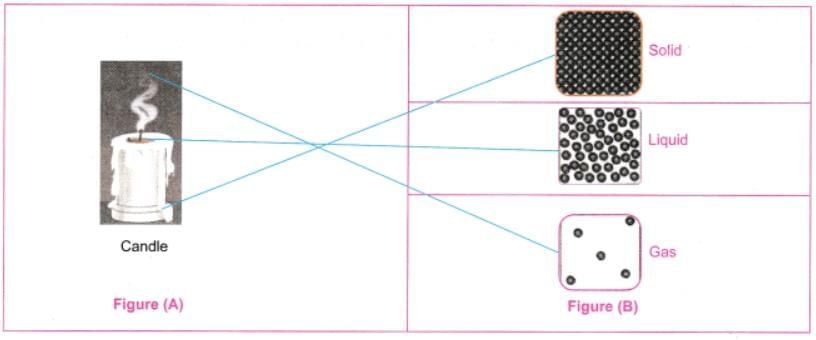
- Solid wax (at base): Rigid, unmelted portion—matches tightly packed particles.
- Liquid wax (melted pool): Flowing around wick—matches loosely arranged particles with movement.
- Gaseous wax (vapour/smoke): Rising as fumes—matches widely spaced, freely moving particles.
The figure shows transitions: solid to liquid (melting) and liquid to gas (evaporation), with particle arrangements changing from fixed to mobile.
Q9: Why does the water in the ocean taste salty, even though the salt is not visible? Explain.
Ans: Ocean water tastes salty because it contains a high concentration of dissolved salts, primarily sodium chloride (common table salt). These salts are not visible because they are dissolved at a molecular level, meaning the individual salt molecules are dispersed throughout the water, making it appear clear.
Q10: Grains of rice and rice flour take the shape of the container when placed in different jars. Are they solids or liquids? Explain.
Ans: Grains of rice and rice flour are considered solids, despite appearing to take the shape of their container. This is because each grain retains its shape and volume, even when mixed. The “flowing” behavior is due to the ability of these small, irregularly shaped particles to move past each other with minimal friction.
Discover, Design, and Debate (Page 115)
Q1: Fix a balloon over the neck of a bottle and put the bottle in hot water. Explore what will happen?
Ans: The balloon inflates. Hot water heats the air inside the bottle, increasing the kinetic energy of air particles, causing them to move faster and spread out (expand). This raises the pressure inside, pushing air into the balloon. It demonstrates gas particles' response to heat, with increased motion and spacing.
Q2: Design and create simple models to represent particles of solids, liquids, and gases showing interparticle spacing using clay balls, beads, etc.
Ans:
- Solid: Arrange clay balls in a tight grid (e.g., in a box), touching each other to show minimal spacing and fixed positions.
- Liquid: Place beads in a shallow tray, close but movable when shaken, illustrating slight spacing and flow.
- Gas: Scatter beads loosely in a large container, shaking to show random motion and maximum spacing.
These models visualize how interparticle spacing increases from solids to gases, affecting properties like shape and volume.
Q3: Pretend to be particles of solids, liquids, and gases, at different temperatures—create and perform a role-play/dance showing particles in motion.
Ans:
- Solid (low temperature): Students stand close, holding hands tightly, only vibrating in place (small shakes).
- Liquid (medium temperature): Loosen grips, move arms while sliding around in a group, showing flow but staying somewhat together.
- Gas (high temperature): Break apart, run freely in all directions with energetic jumps, simulating random motion.
Increase "temperature" by speeding up movements to show phase changes, like "melting" from solid to liquid.
Q4: Debate in the class — ‘Gases can spread and fill all the available space’. Is this property of gases beneficial or harmful?
Ans:
- Beneficial side: Gases spreading enables essential processes like oxygen diffusion in breathing, fragrance dispersal in perfumes, and even weather patterns (wind). It aids in cooking (gas stoves) and inflation (tires/balloons), making life convenient and supporting ecosystems.
- Harmful side: It can spread pollutants or toxic gases quickly (e.g., air pollution, gas leaks causing accidents), leading to health risks or environmental damage like greenhouse gases contributing to climate change.
Overall, the property is mostly beneficial when controlled but harmful if unmanaged—debate could conclude with the need for safety measures like ventilation.
|
59 videos|236 docs|13 tests
|
FAQs on Particulate Nature of Matter NCERT Solutions - Science Curiosity Class 8 - New NCERT
| 1. What is the particulate nature of matter? |  |
| 2. How do particles behave in different states of matter? |  |
| 3. What are the main differences between atoms and molecules? |  |
| 4. Can you explain how temperature affects the movement of particles? |  |
| 5. What role do intermolecular forces play in the behavior of matter? |  |
















