Long Answer Type Questions: Nature of Matter: Elements, Compounds, and Mixtures | Science Curiosity Class 8 - New NCERT PDF Download
Long Answer Type Questions
Q1. Under which category of mixtures will you classify alloys and why?
Answer: When constituent particles of a combination of two or more element or compound retains their properties, then it is called mixture. In an alloy the constituent particles, hence alloys are classified as mixture. For example; steel is an alloy of carbon and iron.
Q2. Iron filings and sulphur were mixed together and divided into two parts, ‘A’ and ‘B’. Part ‘A’ was heated strongly while Part ‘B’ was not heated. Dilute hydrochloric acid was added to both the Parts and evolution of gas was seen in both the cases. How will you identify the gases evolved?
Answer:
In a 20% solution containing 100 g water; the mass percentage of water = 100 – 20 = 80%
∴ 80% of solution is 100 gm
∴ 100% of solution is 100/80 gm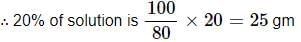
Hence; to prepare 20% (w/w) solution in 100 gram of water 25 gram of sodium sulphate is needed.
Q3. Classify each of the following, as a physical or a chemical change. Give reasons.
(a) Drying of a shirt in the sun.
Answer: Drying of shirt in the sun is a Physical change. Since in this change no new substance is formed.
(b) Rising of hot air over a radiator.
Answer: Since, in rising of hot air over a radiator no new substance is formed, hence it is a Physical change.
(c) Burning of kerosene in a lantern.
Answer: While burning of kerosene in a lantern carbon dioxide, and water vapour is formed, hence it is a Chemical change.
(d) Change in the colour of black tea on adding lemon juice to it.
Answer: In this change a new substance is formed, hence it is a Chemical change.
(e) Churning of milk cream to get butter.
Answer: While churning of milk cream to get butter, no new substance is formed, hence it is a Physical change.\
Q4. Why is air considered a uniform mixture, and how can the presence of carbon dioxide in air be confirmed experimentally?
Answer:
- Air is a uniform mixture because its gases, such as nitrogen (78%), oxygen (21%), and carbon dioxide, are evenly distributed and cannot be seen separately, even with a microscope, as each gas retains its properties.
- To confirm the presence of carbon dioxide, lime water (calcium hydroxide solution) is exposed to air in a petri dish.
- The lime water turns milky due to the formation of calcium carbonate, an insoluble white substance, when carbon dioxide reacts with it.
- This reaction proves carbon dioxide is a component of air. The experiment also shows that exhaled air turns lime water milky faster due to higher carbon dioxide content.
Q5. How does heating a mixture of iron filings and sulfur powder create a new substance, and what tests confirm this change?
Answer:
- Heating a mixture of iron filings and sulfur powder forms iron sulfide, a black compound with new properties, unlike the original mixture where iron is magnetic and sulfur is yellow.
- In the mixture (Sample A), a magnet attracts iron filings, but iron sulfide (Sample B) is not magnetic, showing a chemical change. When dilute hydrochloric acid is added, the mixture produces hydrogen gas (pop sound with a burning splinter), while iron sulfide produces hydrogen sulfide gas (rotten egg smell).
- These tests confirm that iron and sulfur chemically combine to form a new substance. The compound cannot be separated back into iron and sulfur by physical methods.
Q6. Why is water classified as a compound and not a mixture, and how does passing electricity through water support this?
Answer:
- Water is a compound because hydrogen and oxygen are chemically bonded in a fixed 2:1 ratio, creating a new substance with different properties, like extinguishing fire, unlike its flammable elements.
- When electricity is passed through water with a few drops of sulfuric acid, it breaks down into hydrogen and oxygen gases in a 2:1 volume ratio, collected in test tubes.
- The hydrogen produces a pop sound with a burning candle, and oxygen makes the flame glow brighter. This shows water is made of chemically combined elements, not a mixture separable by physical methods.
- The fixed ratio and unique properties confirm water’s status as a compound.
Q7. How do mixtures differ from compounds, using sprout salad and sodium chloride as examples?
Answer:
- Mixtures, like sprout salad, consist of components such as green gram, chickpeas, and tomato that retain their individual properties, are visible, and can be separated physically, like by handpicking, as they do not chemically react.
- Sodium chloride, a compound, is formed by sodium and chlorine chemically combining in a 1:1 ratio, creating a new substance with different properties (taste-enhancing, harmless) unlike its elements (soft metal, hazardous gas).
- Mixtures have variable compositions, while compounds have fixed ratios. Sprout salad is non-uniform, while sodium chloride’s particles are identical throughout. Physical methods cannot separate sodium chloride into sodium and chlorine, unlike sprout salad’s components.
Q8. What are elements, and how do they differ from compounds in terms of composition and properties, using oxygen and water as examples?
Answer:
- Elements are pure substances made of one type of atom, like oxygen, which cannot be broken down further and exists as molecules (O₂) with specific properties, such as supporting combustion.
- Compounds, like water, are formed when elements (hydrogen and oxygen) combine chemically in a fixed 2:1 ratio, creating a new substance with different properties, such as extinguishing fire.
- Oxygen’s atoms are identical, while water contains two types of atoms bonded together. Elements retain their unique traits, but compounds have properties distinct from their constituent elements.
- This difference is evident in water’s inability to be separated physically, unlike mixtures.
Q9. How are alloys like brass useful in daily life, and why are they considered mixtures rather than compounds?
Answer:
- Brass, an alloy of copper and zinc, is useful for making utensils and decorative items because it is stronger and more durable than pure copper or zinc.
- It is a uniform mixture because its components are evenly blended, retaining their individual properties, and can vary in proportion (e.g., 30% zinc, 70% copper).
- Unlike compounds, which have fixed ratios and new properties due to chemical bonding, brass does not involve chemical reactions between copper and zinc. Its uniform appearance and enhanced strength make it practical for everyday use.
- This distinction shows why alloys are mixtures, not compounds.
Q10. Why is sugar a compound, and what happens when it is heated strongly to demonstrate this?
Answer:
- Sugar is a compound because it is made of carbon, hydrogen, and oxygen chemically combined, with properties different from its elements.
- When sugar is heated strongly in a boiling tube, it turns brown, then black, forming carbon (charcoal) and releasing water droplets. These water droplets come from the sugar, not the air, indicating it contains hydrogen and oxygen.
- The black residue (carbon) confirms sugar is not an element but a compound of multiple elements. This decomposition shows sugar’s chemical composition and fixed structure.
|
54 videos|256 docs|13 tests
|
FAQs on Long Answer Type Questions: Nature of Matter: Elements, Compounds, and Mixtures - Science Curiosity Class 8 - New NCERT
| 1. What is the difference between elements, compounds, and mixtures? |  |
| 2. How can we separate components of a mixture? |  |
| 3. Can compounds be broken down into simpler substances? If so, how? |  |
| 4. What are the characteristics of elements? |  |
| 5. How are mixtures classified? |  |
















