Short and Long Answer Questions: Particulate Nature of Matter | Science Curiosity Class 8 - New NCERT PDF Download
Short Answer Type Questions
Q.1. Explain why solids have a fixed shape but liquids and gases do not have a fixed shape.
Ans. Solids maintain a fixed shape because of strong intermolecular forces that hold their particles tightly together. In contrast:
- Liquids have weaker intermolecular forces, allowing their particles to move freely. This enables them to flow and take the shape of their container.
- Gases have even weaker forces, resulting in particles that are far apart and move rapidly. They expand to fill any available space.
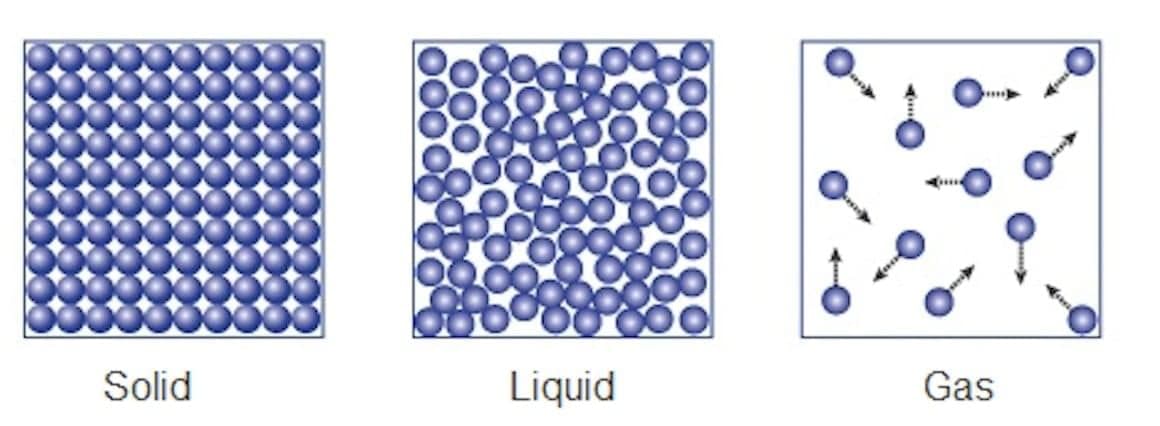
Thus, solids are rigid and retain their shape, while liquids and gases are fluid and adaptable to their surroundings.
Q.2. Liquids and gases can be compressed but it is difficult to compress solids. Why?
Ans. Liquids and gases can be compressed, while solids cannot. This is due to the following reasons:
- Intermolecular space: Liquids and gases have space between their molecules, allowing them to be pushed closer together when pressure is applied.
- Solid structure: Solids have tightly packed molecules with little to no space between them, making it difficult to compress.
In summary, the lack of intermolecular space in solids prevents them from being compressed effectively.
Q.3. What are fluids?
Ans. Fluids are substances that can flow and take the shape of their container. They include:
- Liquids - Have a definite volume but no fixed shape.
- Gases - Have neither a fixed shape nor a fixed volume.
This ability to flow is due to the weaker intermolecular forces present in these states of matter.
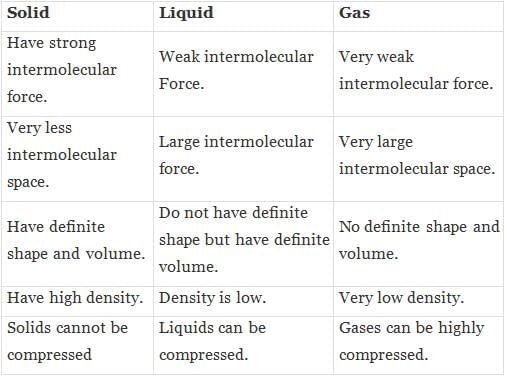
Q.4. State the differences between solid, liquid and gas.
Ans.
Long Answer Type Questions
Q1: Explain the interconversion of three states in terms of force of attraction and kinetic energy of the molecules.
Ans: Matter exists in three main states—solid, liquid, and gas. These states can change into one another by varying temperature and pressure. These changes are based on two key properties of particles:
- Intermolecular Force of Attraction
- Kinetic Energy
1. Solids:
In solids, the particles are tightly packed due to strong forces of attraction. The kinetic energy is very low, so particles only vibrate at fixed positions.
2. Liquids:
When a solid is heated, its particles gain energy and vibrate more vigorously. At a certain temperature (melting point), the force of attraction is weakened, and the solid becomes a liquid. Particles in liquids have more kinetic energy and can slide past each other.
3. Gases:
Further heating of the liquid increases kinetic energy so much that the particles overcome almost all the attraction and move freely. The liquid changes into gas (boiling point). Gases have the weakest forces of attraction and the highest kinetic energy.
Hence, increasing temperature or decreasing pressure leads to a change of state by altering the kinetic energy and intermolecular attraction among particles. Interconversion of three states
Interconversion of three states
Changes in temperature or pressure can cause these states to convert into one another:
- Sublimation: Solid to gas without becoming liquid.
- Deposition: Gas to solid without becoming liquid.
- Boiling: Liquid to gas throughout the liquid.
- Evaporation: Liquid to gas from the surface.
Q2: The melting point of ice is 273.15 K. What does this mean? Explain in detail.
Ans: The melting point of a solid is the temperature at which it changes into a liquid at normal atmospheric pressure. For ice, this melting point is 273.15 K (0°C).
Explanation:
- At 273.15 K, ice starts to melt into water.
- The temperature remains constant during this process, even though heat is continuously supplied.
- The heat energy is used to overcome the strong intermolecular forces holding the solid particles together.
- This energy is called the latent heat of fusion.
- Once all the ice melts, the temperature of the resulting liquid water starts to rise if more heat is supplied.
The melting point of ice signifies the temperature at which it turns into liquid water without any change in temperature, by absorbing latent heat.
|
59 videos|236 docs|13 tests
|
FAQs on Short and Long Answer Questions: Particulate Nature of Matter - Science Curiosity Class 8 - New NCERT
| 1. What is the particulate nature of matter? |  |
| 2. How do the properties of solids, liquids, and gases differ based on their particulate nature? |  |
| 3. What are some historical experiments that supported the particulate nature of matter? |  |
| 4. How does temperature affect the movement of particles in matter? |  |
| 5. Why is understanding the particulate nature of matter important in daily life? |  |





















