Unit Test Solutions: Particulate Nature of Matter | Science Curiosity Class 8 - New NCERT PDF Download
Time: 1 hour
M.M. 30
Attempt all questions.
- Question numbers 1 to 5 carry 1 mark each.
- Question numbers 6 to 8 carry 2 marks each.
- Question numbers 9 to 11 carry 3 marks each.
- Question numbers 12 & 13 carry 5 marks each.
- 1-mark questions include MCQs.
Chapter 7: Particulate Nature of Matter — Unit Test with Detailed Solutions
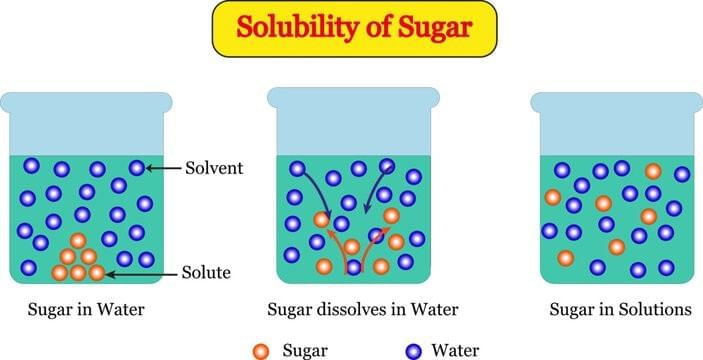
Q1: Which statement best explains why sugar “disappears” when dissolved in water? (1 Mark)
(i) Sugar is destroyed during stirring.
(ii) Sugar changes into air bubbles.
(iii) Sugar breaks into tiny constituent particles that spread in spaces between water particles.
(iv) Water particles convert sugar into water.
Ans: (iii)
Sugar does not vanish; it separates into extremely small constituent particles that occupy the interparticle spaces of water, making a uniform solution while retaining sweetness.
Q2: Solids have a definite shape mainly because (1 Mark)
(i) their particles are far apart.
(ii) their interparticle attractions are very strong and particles vibrate about fixed positions.
(iii) their particles move freely in all directions.
(iv) they have no particles.
Ans: (ii)
In solids, particles are closely packed with maximum interparticle attraction; they can only vibrate about fixed positions, giving fixed shape and volume.
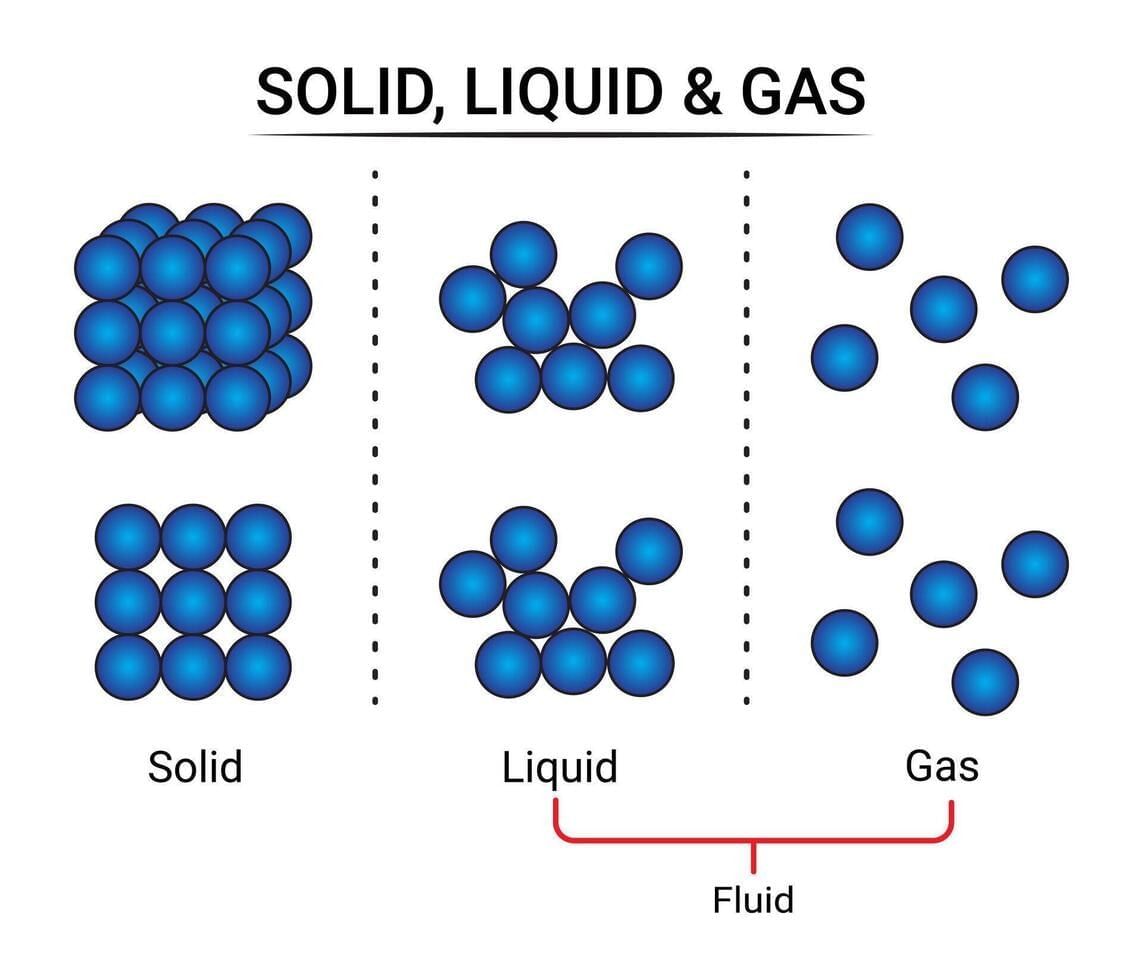
Q3: Which pair correctly matches the state with particle spacing? (1 Mark)
(i) Solid — maximum spacing
(ii) Liquid — negligible spacing
(iii) Gas — maximum spacing
(iv) Solid — particles free to move throughout
Ans: (iii)
Gases have the largest interparticle spacing with negligible attractions; liquids have intermediate spacing; solids have minimum spacing.
Q4: The temperature at which a solid changes into a liquid at atmospheric pressure is called the (1 Mark)
(i) boiling point
(ii) melting point
(iii) condensation point
(iv) sublimation point
Ans: (ii)
Melting point is the minimum temperature at which a solid becomes liquid at atmospheric pressure due to weakened interparticle attractions.
Q5: Smoke/iodine vapour filling an inverted gas jar demonstrates that gases (1 Mark)
(i) have fixed volume
(ii) do not move
(iii) expand to occupy available space and take container’s shape
(iv) only rise upward
Ans: (iii)
Gas particles move randomly in all directions with negligible attractions, so a gas spreads to fill the entire available space and takes the vessel’s shape.
Q6: State two observations from classroom activities that support “matter is made of extremely small particles.” (2 Marks)
Ans:
(i) Grinding chalk gives ever smaller specks, each still chalk, showing larger pieces consist of many smaller constituent particles.
(ii) Sugar dissolves yet cannot be seen, but solution tastes sweet—sugar particles are too small to see and disperse among water particles.
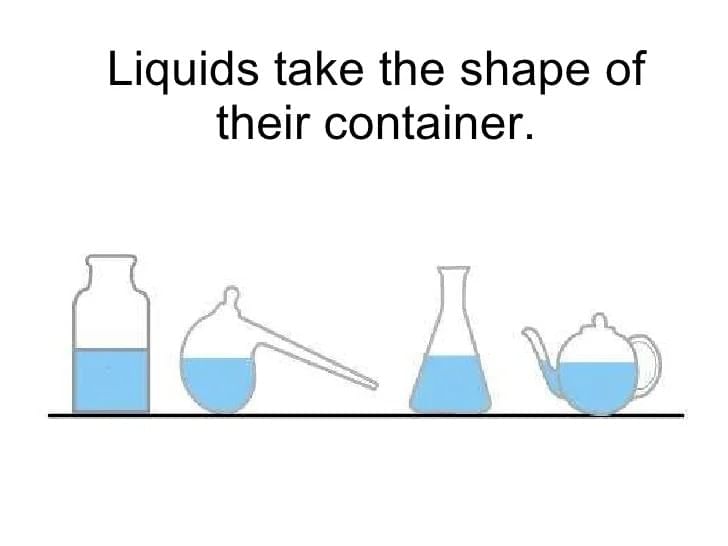
Q7: Explain why liquids have a definite volume but no fixed shape, using particle ideas. (2 Marks)
Ans: Liquid particles have weaker attractions than solids and more spacing; they remain close (giving fixed volume) but can slide past one another (so they adopt the container’s shape rather than keeping their own).
Q8: A syringe without a needle is filled with air and sealed by a thumb; pushing the plunger makes the volume smaller. What does this reveal about gases? (2 Marks)
Ans: Gas particles have large interparticle spaces; under applied pressure, particles can be forced closer, so gases are compressible—unlike liquids which are practically incompressible.
Q9: Differentiate among solids, liquids, and gases based on
(a) interparticle attraction,
(b) interparticle spacing,
(c) particle motion. (3 Marks)
Ans:
(a) Attraction: maximum in solids; moderate in liquids; negligible in gases.
(b) Spacing: minimum in solids; more in liquids; maximum in gases.
(c) Motion: solids—vibrations about fixed positions; liquids—free to move within limited volume; gases—free motion in all available space.
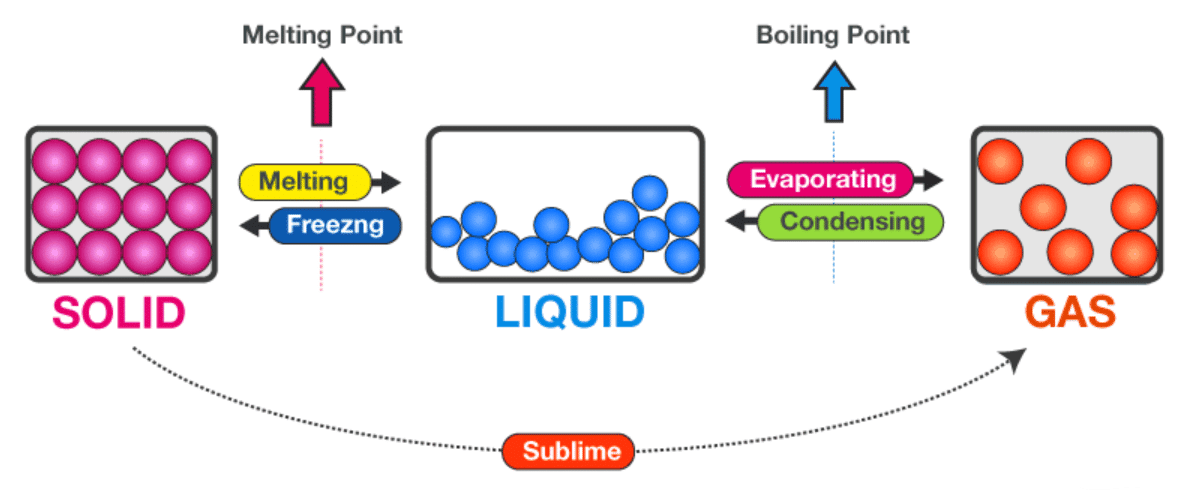
Q10: Define melting point and boiling point; describe particle-level changes at each. (3 Marks)
Ans:
Melting point: temperature where a solid becomes liquid at atmospheric pressure; vibrations increase until particles overcome some attractions and leave fixed positions.
Boiling point: temperature where a liquid rapidly converts to vapour throughout the bulk; particle energy is sufficient to overcome attractions, forming bubbles as particles escape to gaseous state.
Q11: In a clean vessel, water at 200 mL receives two spoons of sugar; level rises then after dissolving, it slightly falls below the mark. Explain. (3 Marks)
Ans: Initially, adding undissolved solid increases apparent volume. Upon dissolution, sugar’s tiny particles occupy interparticle spaces among water particles, so final solution volume is less than water volume + sugar’s bulk volume, causing a slight drop relative to the initial rise.
Q12:
(a) With suitable labeled sketches, explain how particle arrangement changes when ice melts and then boils into steam.
(b) Why does potassium permanganate spread fastest in hot water compared to cold water?
(c) Sand does not dissolve in water. Using particles, explain the observed volume change when sand is added to water. (5 Marks)
Ans:
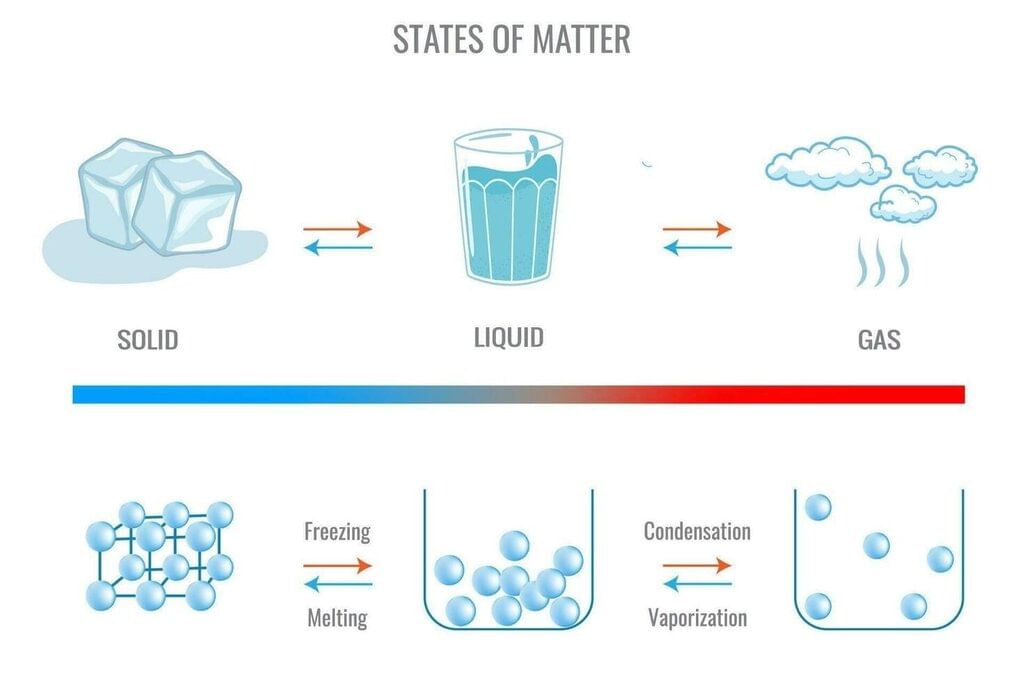
(a)
- Solid (ice): particles closely packed in fixed positions; strong attractions; only vibrations.
- Liquid (water): particles still close but disordered; weaker attractions; can slide past one another.
- Gas (steam): particles far apart; negligible attractions; rapid random motion filling space.
(b) Heating increases particles’ kinetic energy; faster-moving water particles pull out and disperse solute particles more quickly, so diffusion and mixing occur faster in hot water.
(c) Sand’s particles are held together too strongly and are insoluble; they don’t enter water’s interparticle spaces but occupy their own space in the container, so total volume increases (water + sand voids). Some water enters pores between sand grains, so increase is less than sand’s loose bulk volume.
Q13: Answer the following application questions.
(a) Gases mix easily whereas solids usually do not—justify using interparticle attractions and spacing.
(b) Milk spreads when spilled but the glass cup retains its shape—explain.
(c) “If all constituent particles were removed from a chair, nothing of the chair would remain.” Defend this statement. (5 Marks)
Ans:
(a) Gas particles have maximum spacing and negligible attractions; they move freely, so different gases intermix (diffuse) rapidly. Solids have strong attractions and minimal spacing; particles are locked in place, so bulk solids do not mix at the particle level without special processes (e.g., grinding).
(b) Milk is a liquid: particles can move and flow within a limited volume, so it spreads and takes the surface’s shape. The glass is a solid with fixed particle positions and strong attractions, so it keeps its own shape.
(c) All observable matter is composed of constituent particles (atoms/molecules). Removing every particle removes the substance itself—no particles means no material remains, so the “chair” ceases to exist.
|
59 videos|235 docs|13 tests
|
FAQs on Unit Test Solutions: Particulate Nature of Matter - Science Curiosity Class 8 - New NCERT
| 1. What is the particulate nature of matter? |  |
| 2. How does temperature affect the movement of particles in matter? |  |
| 3. What distinguishes solids, liquids, and gases based on the arrangement of particles? |  |
| 4. Can you explain the process of diffusion in relation to the particulate nature of matter? |  |
| 5. Why is the understanding of the particulate nature of matter important in science? |  |
















