MCQ and Extra Questions - Particulate Nature of Matter | Science Curiosity Class 8 - New NCERT PDF Download
Multiple Choice Questions (MCQs)
Question 1:
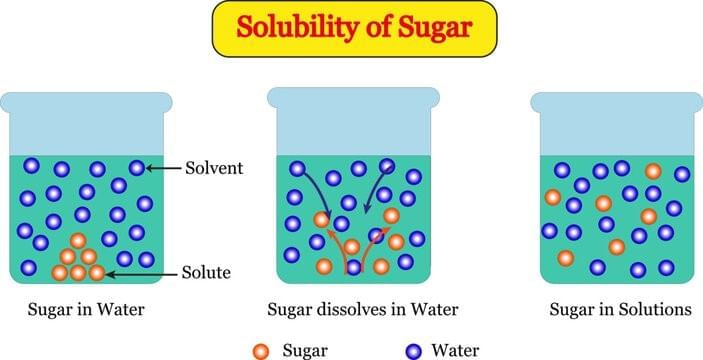
Why does sugar “disappear” when dissolved in water but the water tastes sweet?
Option A: Sugar breaks into tiny constituent particles that occupy interparticle spaces of water
Option B: Sugar converts into water upon stirring
Option C: Water destroys the taste of sugar molecules
Option D: Sugar floats to the top and becomes invisible
 View Answer
View Answer 
Answer: Option A
Solution:
B – Stirring doesn’t turn sugar into water
C – Taste remains
D – Sugar spreads inside water, not float on top
Question 2:
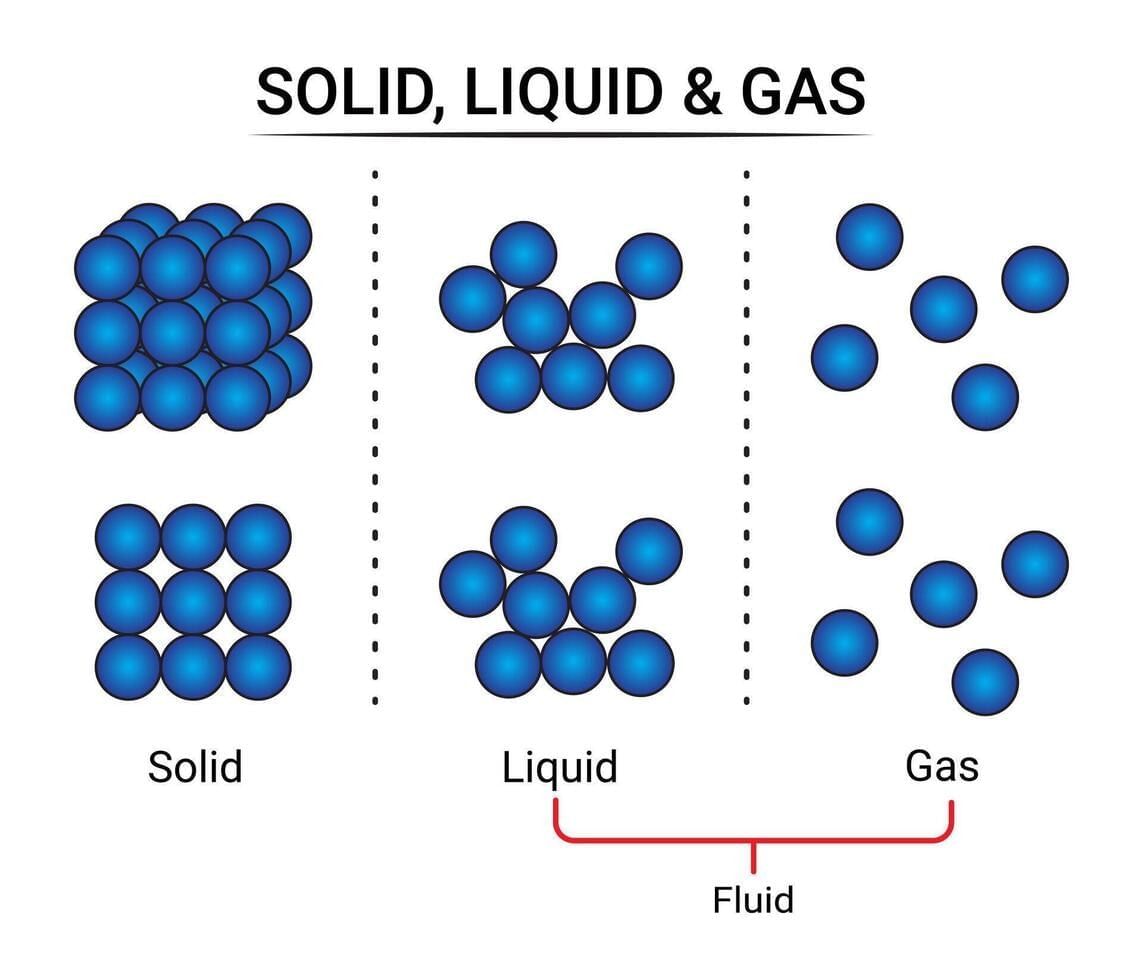
Which feature primarily gives solids a fixed shape and volume?
Option A: Zero interparticle attractions
Option B: Strong interparticle attractions with minimal interparticle spacing
Option C: Particles moving freely in all directions
Option D: Particles having no mass
 View Answer
View Answer 
Answer: Option B
Solution:
- In solids, particles are tightly packed with strong attractions. They only vibrate in place, giving solids a fixed shape and size.
- Why others are incorrect: A and C describe gases, not solids; D is untrue—particles have mass.
Question 3:
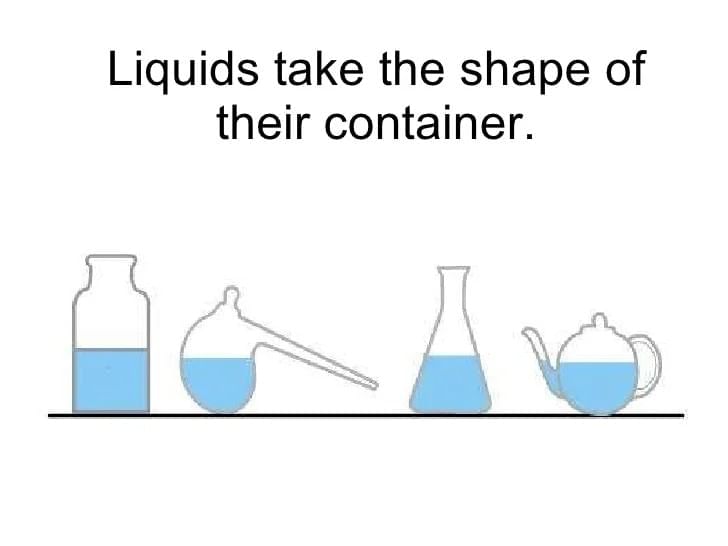
Liquids take the shape of their container but maintain a fixed volume because their particles:
Option A: Are fixed in place like solids
Option B: Have negligible attractions and huge separations
Option C: Can move past each other within a limited space
Option D: Continuously convert into gas at all temperatures
 View Answer
View Answer 
Answer: Option C
Solution:
- Liquid particles have weaker attractions than solids and can slide past each other while remaining close, yielding no fixed shape but definite volume.
- Why others are incorrect: A is solid-like; B fits gases; D confuses slow surface evaporation with continuous full conversion.
Question 4:
Which statement best distinguishes gases from liquids and solids?
Option A: Gases do not have particles
Option B: Gas particles have maximum interparticle spacing and move freely in all available space
Option C: Gas particles are fixed but far apart
Option D: Gases have fixed shape and variable volume
 View Answer
View Answer 
Answer: Option B
Solution:
- Gases exhibit minimal interparticle attractions and maximum spacing, so particles move freely to fill any container, lacking fixed shape or volume.
- Why others are incorrect: A is false—gases are particulate; C mixes properties; D contradicts gas behavior.
Question 5:
During melting of a solid at its melting point (atmospheric pressure), the key microscopic change is:
Option A: Interparticle attractions become stronger
Option B: Particles lose all energy and stop moving
Option C: Particle vibrations increase enough to overcome attractions and move past each other
Option D: Particles convert into different substances
 View Answer
View Answer 
Answer: Option C
Solution:
- Heating makes particles vibrate faster. At the melting point, they overcome strong attractions and move around — forming a liquid.
- Why others are incorrect: A is opposite; B contradicts heating; D is false—melting is a physical change, not chemical.
Question 6:
Which observation best supports the presence of large interparticle spaces in gases?
Option A: A brick keeps its shape
Option B: Incense fragrance spreads across a room
Option C: Sugar forms a solution in water
Option D: A metal nail is rigid and hard
 View Answer
View Answer 
Answer: Option B
Solution:
- The rapid spread of fragrance shows gas particles move freely, colliding and carrying scent molecules through large available spaces.
- Why others are incorrect: A and D describe solids; C demonstrates dissolution and interparticle spaces in liquids, not gases.
Question 7:
When air in a syringe (with the tip blocked) is compressed by pushing the plunger, it compresses because:
Option A: Interparticle spaces decrease under applied pressure
Option B: Gas particles shrink in size
Option C: Gas converts to solid instantly
Option D: The syringe removes particles
 View Answer
View Answer 
Answer: Option A
Solution:
- Gases are highly compressible since most of their volume is empty space between particles; applying pressure reduces this spacing.
- Why others are incorrect: B particles do not shrink; C is untrue; D does not occur in a sealed syringe.

Question 8:
Which statement correctly compares interparticle attractions across states?
Option A: Solids weakest, liquids medium, gases strongest
Option B: Only gases have attractions
Option C: Same in all three states
Option D: Solids strongest, liquids weaker, gases negligible
 View Answer
View Answer 
Answer: Option D
Solution:
- Attractions: solids > liquids > gases; this ordering correlates with packing, spacing, and mobility across states.
- Why others are incorrect: A reverses the trend; B and C contradict observed behavior.
Question 9:
Which is a correct statement about dissolution of a soluble solid in water?
Option A: The final volume is always the sum of solid volume plus water volume
Option B: Dissolved particles occupy interparticle spaces so the total volume may be less than the simple sum
Option C: Dissolution destroys the solid’s particles
Option D: Solids cannot occupy spaces in liquids
 View Answer
View Answer 
Answer: Option B
Solution:
- Solute particles fit into spaces between water particles, so solution volume can be less than the arithmetic sum, evidencing interparticle spacing in liquids.
- Why others are incorrect: A ignores packing effects; C is false; D contradicts dissolution phenomenon.
Question 10:
Why does sand not dissolve in water while sugar does?
Option A: Sand particles are too large and do not interact favorably with water; sugar particles disperse and interact, fitting into water’s interparticle spaces
Option B: Sand has no particles
Option C: Water has no interparticle spaces
Option D: Sugar melts in water but sand freezes
 View Answer
View Answer 
Answer: Option A
Solution:
- Solubility depends on particle-level interactions; sugar-water interactions allow dispersion into interparticle spaces, whereas sand (insoluble) does not form such interactions and settles.
- Why others are incorrect: B and C are false; D is nonsensical regarding melting/freezing.
Short Answer Questions
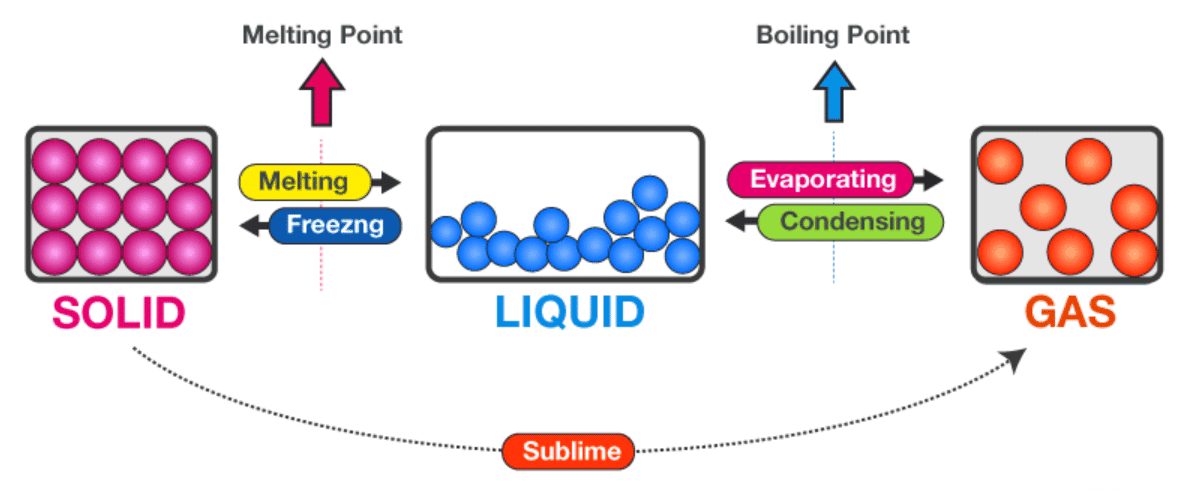
Q1. What happens during melting, and why is it considered a change of state?
Answer:
- Melting occurs when a solid is heated and absorbs thermal energy.
- This energy increases particle vibrations until they can overcome some of the attractive forces holding them together.
- As a result, particles move past one another, and the rigid solid turns into a liquid.
- This is a change of state because the physical form changes, but the substance itself remains the same.
Q2. Which observation shows that liquid particles are mobile but stay close together?
Answer:
- When a finger is dipped into water and removed, the liquid quickly flows back to occupy the space, leaving no permanent cut.
- This shows that liquid particles can slide past each other (mobility) but are still close enough to stick together (cohesion).
This behavior is unique to liquids:
- Solids (like steel or chalk) are rigid and cannot flow.
- Gases spread freely to fill the entire container, unlike liquids which keep a definite volume.
Q3. How does heating affect the motion of particles?
Answer:
Heating increases the kinetic energy of particles in all states of matter.
As temperature rises, particles move faster, causing:
- Increased diffusion (e.g., color from potassium permanganate spreads faster in hot water than in cold water).
- Greater rate of spreading of gases and liquids.
Q14. Which state of matter is highly compressible, and why?
Answer:
- Gases are highly compressible because there are very large empty spaces between particles.
- When pressure is applied, these spaces can be reduced, decreasing the volume of the gas.
- This is why gases can be stored in cylinders under high pressure.
- Solids and liquids, however, have very little empty space between particles and thus cannot be compressed easily.
Q15. What does breaking chalk into powder show about the particulate nature of matter?
Answer:
- When chalk is broken into tiny pieces or powder, it still remains chalk.
- This shows that matter is made of tiny particles that retain the identity of the substance.
- The process is a physical change, as only the size of the pieces changes, not the substance itself.
- This proves that matter has a particulate nature—particles are too small to be seen individually but still make up the same material.
|
59 videos|236 docs|13 tests
|
FAQs on MCQ and Extra Questions - Particulate Nature of Matter - Science Curiosity Class 8 - New NCERT
| 1. What is the particulate nature of matter? |  |
| 2. How do the properties of solids, liquids, and gases differ based on the particulate nature of matter? |  |
| 3. What role did scientists like John Dalton and J.J. Thomson play in the understanding of the particulate nature of matter? |  |
| 4. How does temperature affect the motion of particles in matter? |  |
| 5. Why are gases considered to have no definite shape or volume? |  |
















