Communicable and Non-communicable Diseases - 1 Chapter Notes | Preventive and Social Medicine (PSM) - NEET PG PDF Download
Period of Communicability
- Chickenpox: 1-2 days before and 4-5 days after the rash appears
- Measles: Days before and 5 days after the rash appears
- Rubella: Days before symptoms and 7 days after the rash appears
- Mumps: 4-6 days before symptoms and 7 days after
- Influenza: 1-2 days before and 1-2 days after symptoms start
- Diphtheria: 14-28 days from the onset of the disease
- Pertussis: Days after exposure to 3 weeks after the severe cough stage
- Meningococcus: Until no longer present in nasal and throat discharges
- Tuberculosis: As long as it remains untreated
- Poliomyelitis: 7-10 days before and after symptoms begin
- Hepatitis A: Weeks before and 1 week after jaundice starts
- Hepatitis B: Until HBsAg disappears and anti-HBs appears
- Tetanus: None
Common Gestational Periods for Vertical Transmission of Diseases
- Congenital Varicella: First trimester
- Congenital Rubella: First trimester
- Congenital Parvovirus: Second trimester
- Congenital Syphilis: Third trimester
- Congenital Toxoplasmosis: Third trimester
- Congenital Hepatitis B: Third trimester
- Congenital CMV: Third trimester
- Congenital HIV: During delivery
- Congenital Hepatitis C: During delivery
- Congenital Herpes: During delivery
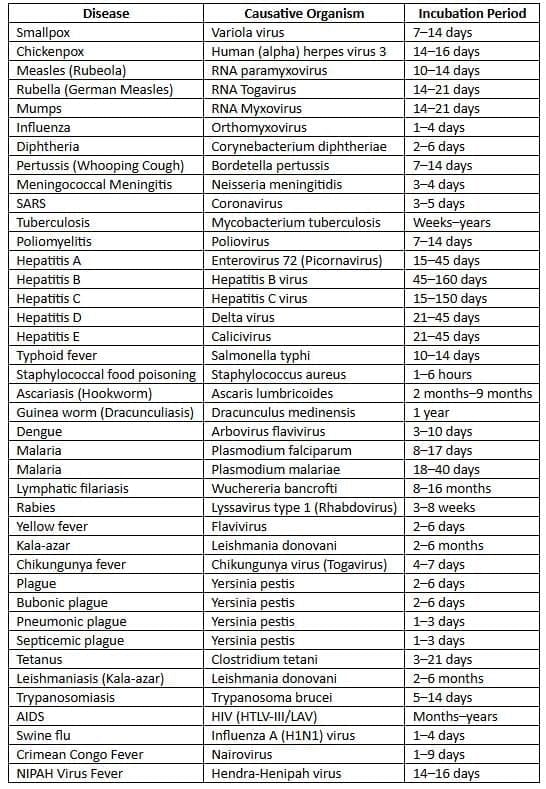
Incubation Periods of Common Diseases
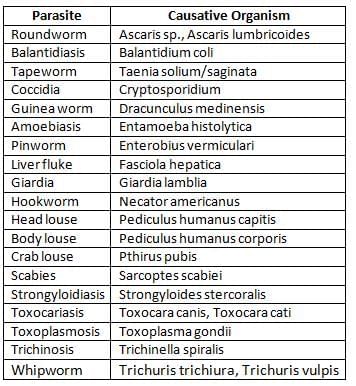
Important Human Parasites
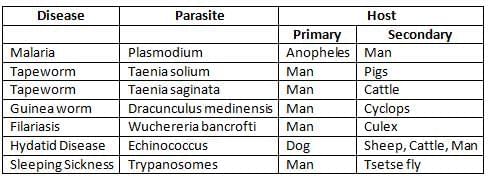
Host of a Disease
- A host refers to a person or animal, including birds and arthropods, that provides food or shelter to an infectious agent under natural conditions.
- Types of Hosts
- Primary (definitive) host: This is the host where the parasite reaches maturity or completes its sexual cycle.
- Secondary (intermediate) host: This host is where the parasite exists in its larval or asexual stage.
- Obligate host: This is the only host for a parasite, such as humans in cases of measles and typhoid fever.
- Transport host: This type of host carries the organism without allowing it to develop, keeping it alive.
- Paratenic host: This is similar to an intermediate host but is not essential for the parasite's life cycle.
- Dead-end host: This is an intermediate host that does not typically transmit to the definitive host, halting the parasite's development.
- For example, humans are dead-end hosts for Echinococcus canine tapeworms.
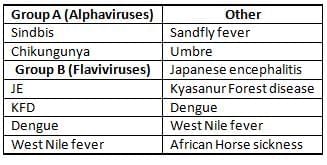
Arboviral Infections in India
Smallpox vs Chickenpox
Smallpox
- Smallpox is characterized by a rash that is pleomorphic, meaning it can present in various forms.
- The rash in smallpox has a centrifugal distribution, spreading from the center of the body to the extremities.
- The rash appears as a dew drop on a rose petal, with vesicles that are superficial and unilocular.
- Smallpox affects flexor surfaces (the inner sides of limbs) and spares the palms and soles.
- Smallpox was eradicated due to several factors:
- No known animal reservoir.
- No long-term carrier state.
- Infection provides lifelong immunity.
- Easy case detection due to the characteristic rash.
- Subclinical cases do not spread the disease.
- Availability of a highly effective vaccine.
- International cooperation in eradication efforts.
Chickenpox
- Also known as Varicella, chickenpox is caused by the Varicella zoster virus, a member of the herpesvirus family.
- The incubation period for chickenpox is typically 14 to 16 days.
- Infection is spread through person-to-person contact, primarily via respiratory droplets.
- Chickenpox is communicable 1 to 2 days before the rash appears and 4 to 5 days after the rash onset.
- The secondary attack rate for chickenpox is around 90%, indicating high transmissibility.
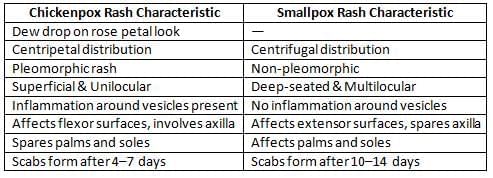
- The rash in chickenpox is non-pleomorphic, meaning it does not vary in appearance like smallpox rash.
- The vesicles in chickenpox can be deep-seated and multilocular, with inflammation surrounding the vesicles.
- Chickenpox affects extensor surfaces (the outer sides of limbs) and involves the palms and soles, unlike smallpox.
- The rash in chickenpox evolves slowly, with scabs forming 10 to 14 days after the rash appears.
- The most common late complication of chickenpox is shingles, which occurs due to the reactivation of the virus years later.
- Diagnosis of chickenpox is most accurately done by examining vesicle fluid under an electron microscope, which shows round viral particles.
- Congenital varicella: where the virus is transmitted from mother to fetus, is most dangerous if contracted during the first trimester of pregnancy.
- Live Attenuated Chickenpox Vaccine
- The live attenuated chickenpox vaccine, using the OKA strain, has a seroconversion rate of over 90%.
- Varicella zoster immunoglobulin (VZIG)
- Can be administered within 72 hours of exposure to chickenpox.
- VZIG is given at a dose of 1.25 to 5.0 ml intramuscularly and is reserved for immunosuppressed contacts of acute cases and newborn contacts.
Measles
- Measles, also known as Rubeola, is caused by a single serotype of RNA paramyxovirus.
- Incubation Period: 10-14 days
- Source of Infection: Measles cases are the source of infection, as carriers are not known to exist.
- Period of Communicability: Measles is communicable 4 days before and 5 days after the rash appears, which typically starts behind the ears.
- Contagiousness: Measles is highly contagious during the early symptoms and when the rash develops.
- Immunity: Individuals cannot get measles more than once, as it provides lifelong immunity.
- Secondary Attack Rate: 80%
- Outbreak Cycle: Measles follows a cycle with outbreaks occurring every 2-3 years.
- Pathognomonic Clinical Feature: Koplik spots, which are found on the inside of the cheeks opposite the upper second molar, are a characteristic feature of measles.
- Most Common Complication in Young Children: Otitis media (ear infection) is the most common complication of measles in young children.
- Rare Complication: Subacute Sclerosing Pan Encephalitis (SSPE) is a rare complication of measles, occurring in 7 per million cases, typically 7-10 years after the initial infection.
- Prevention of Measles:
- Active Immunization: Measles vaccine
- Type: Live, attenuated
- Strains: Edmonston Zagreb (most common), Schwarz, Moraten
- Passive Immunization: Measles immunoglobulin (WHO recommended dose: 0.25 ml/kg body weight)
WHO Measles Elimination Strategy
- Catch up: Nationwide vaccination campaign targeting all children aged 9 months to 14 years, regardless of their measles history or vaccination status.
- Keep up: Routine services aiming to vaccinate over 95% of each new birth group.
- Follow up: National vaccination campaigns every 2-4 years targeting children born after the catch-up campaign.
- Challenges for Measles Elimination
- Weak immunisation systems
- Highly infectious nature of measles
- Inaccessible populations (e.g., those in conflict areas)
- Refusal of immunisation
- Changing patterns of disease (increased spread among adolescents and adults)
- Need to provide catch-up campaigns for over 130 million children in India
- Shortages in human and financial resources at country, regional, and global levels
- Accelerated Measles Mortality Reduction Strategy:
- WHO-UNICEF: Two doses of measles-containing vaccine (MCV) for all children through routine and supplementary immunisation activities.
- Global Measles Elimination Targets by 2015:
- Routine vaccine coverage >90% nationally
- Routine vaccine coverage >80% at district level
- Reduction and annual maintenance of incidence to fewer than 5 cases per million
- Reduce measles mortality by 95%
- Global Measles and Rubella Strategic Plan 2012-2020:
- High population coverage with 2 doses of measles and rubella vaccines
- Effective surveillance
- Preparedness for outbreaks
- Boost public confidence in vaccination
- Research and development
Rubella
Rubella (German Measles)
- Causative agent: RNA virus from the Togavirus family
- Incubation period: 14–21 days (~18 days on average)
- Source of infection:Individuals with active cases or subclinical infections
- No identified carrier state for postnatally acquired rubella
- Mode of transmission: Airborne droplets (respiratory)
- Period of communicability: One week before symptom onset to one week after the rash appears
- Immunity for Rubella:
- A single infection provides lifelong immunity (second attacks are rare)
- 40% of women of reproductive age in India remain susceptible
- Infants are protected up to 4-6 months of age
- Most widely used diagnostic test: Hemagglutination Inhibition test (HAI)
Rubella Vaccine
- Type of vaccine: Live attenuated, strain RA 27/3 [Vaccine virus is non-communicable]
- Dose and route: 0.5 ml, subcutaneous
- Rubella vaccine is not suitable forpregnant women and is not given to infants.
- If a female is vaccinated for rubella: Advice against pregnancy for the next 3 months.
- Priority groups for rubella vaccination in India:
- 1st Priority: Females aged 15 - 49 years
- 2nd Priority: All children aged 1 - 14 years
- 3rd Priority: Routine universal immunization for all children aged 1
Congenital Rubella Syndrome (CRS)
- CRS is indicated if:
- Infant has IgM rubella antibodies shortly after birth, or
- IgG antibodies persist for more than 6 months
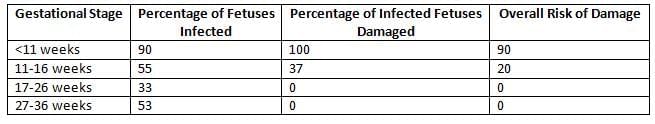
- The extent of fetal infection in CRS mainly depends on the gestational age when the transmission occurs.
- Infection in the 1st trimester: Most Disastrous Time
- Abortions
- Stillbirths
- Skin lesions: blueberry muffin lesions
- The triad of congenital rubella syndrome includes congenital heart defects, with patent ductus arteriosus (PDA) being the most common.
- Cataracts
- Infection in early 2nd trimester: Deafness (only)
- Infection after 16 weeks POG: No major abnormalities
- Risk of fetal harm in Congenital Rubella Syndrome (CRS):
Mumps
- Causative agent: Myxovirus parotiditis (RNA paramyxovirus)
- Incubation Period: 14 - 21 days
- Source of Infection: Clinical & subclinical cases
- Period of Communicability: 4 - 6 days before to 7 days after symptoms start
- Mumps provides life-long immunity.
- Secondary attack rate of Mumps: 86%
- Clinical features: Involvement of salivary glands (especially parotid)
- Most common complication: Aseptic meningitis
- Most common complication in adolescents: Orchitis, Oophoritis
- Mumps prevention: Active immunisation using Mumps vaccine:
- Type: Live attenuated vaccine
- Strain: Jeryll Lynn strain
Influenza
- Causative Agent: Influenza is caused by the Orthomyxovirus, which has three types: A, B, and C.
- Type A: This type is primarily responsible for outbreaks, epidemics, and pandemics.
- Type B: This type causes outbreaks but is less common than Type A.
- Type C: This type is not currently circulating.
- Currently circulating influenza viruses worldwide:
- H1N1 (Type A): Responsible for Swine flu
- H2N2 (Type A):
- H5N1 (Type A): Known for causing avian influenza, or birdflu.
- H3N2
- H7N9
- (Type B): A strain of Type B influenza.
- Cyclical Trends in Influenza:
- Type A epidemics occur every 2 to 3 years.
- Type B epidemics occur every 4 to 7 years.
- Type A pandemics happen every 10 to 15 years.
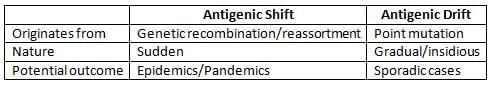
Antigenic Variations in Type A Influenza
- Incubation Period: The incubation period for influenza is typically between 18 to 72 hours.
- Period of Infectivity: Individuals are infectious 1 to 2 days before and 1 to 2 days after the onset of symptoms.
Avian Influenza
- Avian Influenza, commonly known as "Bird flu" or "Highly Pathogenic Avian Influenza," is caused by the H5N1 strain of the (Type A Influenza virus.)
- This disease is a pandemic that originated in Hong Kong in 1997.
- The drug of choice for treatment is Oseltamivir (Tamiflu) at a dosage of 75 mg twice daily for five days, although it is not suitable for infants.
Influenza A (H1N1) pdm 09
- Influenza A (H1N1) pdm 09 was declared a pandemic by the WHO on June 11, 2009.
- Currently, the world is post-pandemic, except for India and New Zealand, where there is locally intense transmission.
- In India, between May 2009 and August 2010, there were 37,000 reported cases and 1,833 deaths.
- Incubation Period: 2-3 days
- Symptoms of Avian Influenza:
- Uncomplicated Influenza: Symptoms include fever, cough, sore throat, runny nose, headache, muscle pain, and gastrointestinal illness such as diarrhea without dehydration.
- Complicated/Severe Influenza: Symptoms may progress to pneumonia, central nervous system involvement, severe diarrhea, secondary complications, and exacerbation of chronic diseases.
- Progressive Disease: This stage is characterized by oxygen impairment, cardiopulmonary insufficiency, central nervous system complications, invasive secondary bacterial infections, and severe dehydration.
- Risk Factors for Severe Disease:
- Infants and children under 2 years old
- Pregnant women
- Individuals with Chronic Obstructive Pulmonary Disease (COPD)
- Those with chronic cardiac diseases
- People with metabolic disorders
- Individuals with chronic renal, hepatic, neurological disorders, hemoglobinopathies, or immunosuppression, including HIV
- Children on aspirin therapy
- Elderly individuals over 65 years old
- Individuals with morbid obesity
Laboratory Diagnosis:
- Most Timely and Sensitive Detection: RT-PCR test is the most timely and sensitive method for detecting Avian Influenza.
- Samples: Nasopharyngeal and throat swabs are commonly used for testing. In cases of lower respiratory tract infection, tracheal or bronchial aspirates may be collected.
- Point-of-Care/Rapid Diagnostic Tests: These tests are not recommended for diagnosing Avian Influenza.
- Duration of Isolation: Patients should be isolated for 7 days after the onset of illness or 24 hours after the resolution of fever and respiratory symptoms, whichever is longer.
- Antiviral Therapy:
- Severe/Progressive Clinical Illness: Oseltamivir is recommended. If not available or if there is resistance, Zanamivir can be used as an alternative.
- High Risk of Severe/Complicated Illness: Both Oseltamivir and Zanamivir are suitable for treatment.
- Not High Risk or Uncomplicated Illness:Treatment is not necessary for individuals who are not at high risk or have uncomplicated confirmed or suspected cases.
- Oseltamivir 75 mg twice daily for 5 days
- Dosage for Zanamivir 2 inhalations (2 x 5 mg) twice daily for 5 days
Avian Influenza H7N9
- Origin: The H7N9 strain of Avian Influenza originated in China in 2013 and later spread to Hong Kong.
- Disease Burden:
- Cases: 141
- Deaths: 45
- Case Fatality Rate: 33%
- Most Affected Age Group: Older males, particularly those over 50 years of age.
- Mode of Transmission: Primarily through respiratory routes, often associated with live bird markets.
- Human-to-human transmission is rare but possible.
- Treatment: Neuraminidase inhibitors, such as Oseltamivir, are used for treatment.
Vaccines for Influenza
- Killed Vaccines:
- Administered in doses 3-4 weeks apart, with a dosage of 0.5 ml for individuals over 3 years of age, via subcutaneous injection.
- Demonstrates 90% protective efficacy with a duration of protection ranging from 3 to 6 months.
- Rarely associated with Guillain-Barré Syndrome (GBS).
- Live Attenuated Vaccines:
- Stimulate both local and systemic immunity.
- Face challenges in manufacturing due to antigenic variations.
- Newer Vaccines:
- Split-Virus Vaccine:
- Also known as Sub-virion vaccine, this vaccine is highly purified with lesser side effects.
- However, it is less antigenic, requiring multiple injections, and is particularly useful for children.
- Split-Virus Vaccine:
- Neuraminidase-Specific Vaccine:
- A sub-unit vaccine containing N-antigen, which permits subclinical infection and provides long-lasting immunity.
- Recombinant Vaccine:
- Involves transferring the antigenic properties of a virulent strain to a less virulent strain.
- Contraindications to Inactivated Influenza Vaccines:
- Severe allergy to chicken eggs.
- History of hypersensitivity or anaphylactic reactions to previous vaccines.
- Development of Guillain-Barré Syndrome (GBS) within 6 weeks of vaccination.
- Infants less than 6 months of age.
- Moderate to severe illness with fever.
H1N1 (Swine Flu) Vaccine
- H1N1 Inactivated Vaccine:
- Administered as a single intramuscular injection.
- Strain: A/California/7/2009 (H1N1) V-like strain.
- Storage Temperature: +2° to +8° C.
- Contraindications: History of anaphylaxis, severe reaction, Guillain-Barré Syndrome, infants under 6 months, and moderate-to-severe illness with fever.
- Protective Immunity: Develops after 14 days, but not 100%.
- H1N1 Live Attenuated Vaccine: Nasal spray
- Administered as a nasal spray.
- Side Effects: Rhinorrhea, nasal congestion, cough, sore throat, fever, wheezing, vomiting.
- Priority Groups for Influenza Vaccines:
- Pregnant women.
- Individuals over 6 months with chronic medical conditions.
- Healthy young adults aged 15-49 years.
- Healthy young children.
- Healthy adults aged 49-65 years.
- Healthy adults over 65 years.
Diphtheria
- Causative agent: Corynebacterium diphtheriae, a gram-positive non-motile organism
- Disease prevalence: Diphtheria is widespread in India
- Source of infection:
- Cases or carriers are the main sources of infection; carriers account for 95% of total disease spread
- Nasal carriers are more hazardous than throat carriers
- Carrier rate in a community: 0.5–1%
- Vaccination does not eliminate the carrier state
- Incubation Period: 2–6 days
- Mode of transmission: Droplet infection (primary method), direct contact via skin lesions, and fomites
- Period of Infectivity: 14–28 days from disease onset; longer for carriers
- A case or carrier may be deemed non-infectious when at least two cultures from nose and throat, taken 24 hours apart, test negative
DPT Vaccine
For detailed information, please refer to Chapter 3, Theory.
Schick Test
- The Schick Test is a skin test used to determine a person’s immunity and sensitivity to the diphtheria toxin.
- Dose: The test involves injecting 0.2 ml of Schick test toxin (1/50 MLD) in one arm and 0.2 ml of heat-inactivated toxin in the other arm as a control
- Interpretation of Schick Test
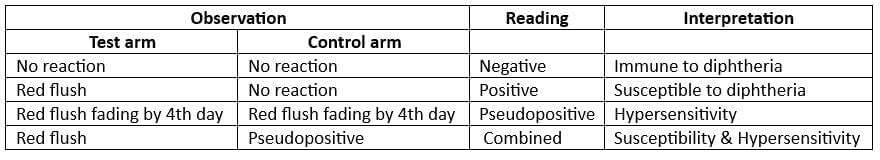
(Red flush = Positive reaction)
- Schick test negative if: >0.03 units of antitoxin per ml in blood serum
- Schick test has been replaced by: Hemagglutination Test
- Hemagglutination Test: Assessment of serum antitoxin levels
Whooping Cough
- Pertussis, commonly known as Whooping Cough or 100 Day Cough, is an infectious disease caused by the bacterium Bordetella pertussis, with a small percentage of cases (about 5%) caused by B. parapertussis.
- The disease is characterized by severe coughing fits that are followed by a high-pitched whooping sound when the affected person inhales.
- Incubation period: The time between exposure to the bacteria and the onset of symptoms is typically between 7 to 14 days.
- Source of infection: Infected individuals are the primary source of the bacteria.
- There is no subclinical or chronic carrier state for this infection, meaning individuals do not carry the bacteria without showing symptoms, nor do they carry it long-term after recovery.
- Both vaccination and previous infection do not provide long-term immunity to pertussis. This means that individuals can become susceptible again after some time.
- The secondary attack rate, which indicates how easily the infection spreads to close contacts, is over 90%. This high rate signifies the contagious nature of the disease.
- Incidence and fatality: Research indicates that pertussis is more common and has a higher fatality rate in females compared to males.
- Leukocytosis: An increase in white blood cells (leukocytosis) does not correlate with the severity of the cough in pertussis cases.
- Chief Complications of Pertussis:
- Bronchitis: Inflammation of the bronchial tubes.
- Bronchopneumonia: A type of pneumonia that affects the bronchi and surrounding lung tissue.
- Bronchiectasis: Permanent dilation of the bronchi, leading to recurrent infections.
- Subconjunctival haemorrhages: Bleeding underneath the conjunctiva of the eye.
- Epistaxis: Nosebleeds.
- Haemoptysis: Coughing up blood.
- Punctate cerebral haemorrhages: Small bleeding spots in the brain.
- Convulsions: Seizures.
- Coma: A state of prolonged unconsciousness.
- Laboratory Diagnosis of Pertussis: Diagnosis can be confirmed by culturing nasopharyngeal swabs on Bordet-Gengou medium, using polymerase chain reaction (PCR), immunofluorescence (DFA), and serological methods.
- Drug of Choice for Pertussis: The recommended treatment is Erythromycin at a dosage of 40 mg/kg, administered four times a day for a duration of 10 days.
- Vaccines for Pertussis: Vaccination against pertussis is included in the DPT vaccine. For more information, refer to Chapter 3, Theory.
Meningococcal Meningitis
- Meningococcal Meningitis, also known as Cerebrospinal Fever, is caused by Neisseria meningitidis, a gram-negative diplococcus.
- There are several serotypes of this bacteria, including A, B, C, D, 29E, W135, X, and Y.
- Meningococcal disease is prevalent in India, where carriers of the bacteria are a more significant source of infection than actual cases.
- The average duration of temporary carriers is about 10 months, and during outbreaks, the carrier rate can increase to 70-80%.
- Mode of Transmission:
- Meningococcal Meningitis is transmitted through droplet infection.
- Incubation Period:
- The incubation period for Meningococcal Meningitis ranges from 2 to 10 days, with an average of 3 to 4 days.
- Case Fatality Rate
- The Case Fatality Rate (CFR) for meningococcal meningitis varies depending on treatment. With early diagnosis and treatment, the CFR can be below 10%.
- Drugs of Choice:
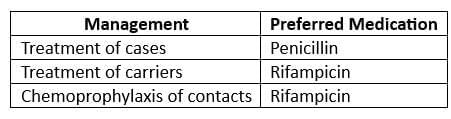
- For chemoprophylaxis: Rifampicin 600 mg BD for 2 days
- Note: Treatment with Penicillin does not eliminate the carrier state.
Meningococcal Vaccine
- Type of Vaccine: Killed vaccine, cellular fraction
- Dose: 0.5 ml
- Route: Subcutaneous
- Site: Antero-lateral thigh, middle one-third
- Booster: Every 3 years
- Available for: Group A, C, W135, and Y meningococci
Group B Meningococcus
- Vaccine is not available for Group B meningococcus.
- Group B polysaccharide vaccines are generally less effective.
- Some vaccines targeting Group B meningococcus are available.
- Contraindications:
- Pregnancy
- Infants and children under 2 years of age (due to the development of immunologic tolerance).
ARI/Pneumonia
No Pneumonia: Cough or Cold
- No chest indrawing, no fast breathing.
- Management:
- Antibiotics are not necessary.
- Treat symptomatically.
- If cough lasts more than 30 days, refer for assessment.
Pneumonia (Not severe)
- Fast breathing is present (based on respiratory rate - RR).
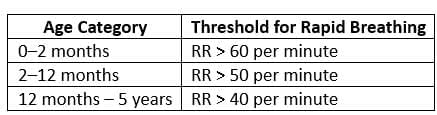 Management:
Management:- At home
- Give antibiotics - Drug of choice: Cotrimoxazole.
- Reassess after 2 days.
Severe Pneumonia
- Signs:
- Chest indrawing
- Nasal flaring
- Grunting
- Cyanosis
- Management:
- Give first dose of referral antibiotic (Ampicillin + Gentamicin).
- Refer Urgently to hospital;
- Drugs of Choice: Benzyl Penicillin (or Ampicillin or Chloramphenicol) for the first 48 hours, then Procaine Penicillin (or Ampicillin or Chloramphenicol) for the next 3 days.
- Antibiotics should be changed if there is no improvement after the first 48 hours.
Very Severe Pneumonia
Signs:
- Convulsions
- Abnormally sleepy or difficult to wake
- Stridor when calm
- Stopped feeding
- Wheezing Fever or low body temperature
- Severe malnutrition
- Management:
- Give first dose of referral antibiotic (Ampicillin + Gentamicin).
- Refer Urgently to hospital;
- Drug of Choice: Chloramphenicol (i/m) for the first 48 hours, then oral Chloramphenicol for a total of 10 days.
- Antibiotics should be changed (to i/m Cloxacillin + Gentamicin) if there is no improvement after the first 48 hours.
Tuberculosis
Tuberculosis Situation in India
- India has the highest burden of Tuberculosis (TB) in the world.
- Two out of five Indians, or 40%, are infected with TB, as indicated by a positive Mantoux test.
- The annual risk of becoming infected with TB in India is 1.5%.
- Among those infected, the lifetime risk of developing the disease is 10%.
- Approximately 5,000 Indians develop TB every day.
- 0.8 million cases are sputum positive for TB each year.
- TB causes 0.37 million deaths per year in India.
Epidemiological Indices for TB
- Incidence of TB infection, also known as the Annual Risk of Infection (ARI), measures the percentage of the population that will be newly infected with TB within a year among those who are not already infected. This index reflects the attacking force of TB within the community. In developing countries, an ARI of 1% corresponds to 50 SS +ve cases per 100,000 people in the general population. The Tuberculin Conversion Index is considered the best indicator for assessing the TB problem and its trend in the community.
- Prevalence of TB infection is the percentage of individuals who show a positive reaction to the standard tuberculin test. This reflects the cumulative experience of the population with both recent and past TB infections. The tuberculin test is the only method for estimating the prevalence of TB infection in a population.
- Incidence of TB disease refers to the percentage of new TB cases per 1000 population. This indicator reveals trends in the TB problem, including the impact of control measures. It is most useful in countries where a high proportion of new cases are detected and reported reliably, with sputum smear examination (AFB) being a reliable estimation method.
- Prevalence of TB disease or case rate indicates the percentage of individuals with sputum positive for TB bacilli on microscopic examination. This is the best practical index for estimating the TB case load in the community, with age-specific prevalence being the most relevant index. Prevalence of suspect cases, based on chest X-ray examinations, has no significant epidemiological value. Prevalence of drug-resistant cases is directly related to chemotherapy practices, while mortality rate was previously used as an index of the TB problem's magnitude.
Tuberculin/PPD
- Tuberculin, also known as purified protein derivative (PPD), has replaced the older tuberculin antigen (OT).
- Tuberculins have been developed from atypical mycobacteria as well:
- PPD-Y (from M. kansasii )
- PPD-B (from Battey mycobacterium )
- Scrofula (from M. scrofulaceum )
- This was discovered by Von Pirquet in 1907.
- PPD is a more refined product, leading to fewer non-specific reactions and greater ease of standardization.
- Standard PPD (PPD-S) contains 50,000 tuberculin units (TU) per mg [1 TU = 0.00002 mg PPD].
- The WHO recommends PPD-RT-23 with Tween-80.
- Dosage:
- First strength (1 TU)
- Intermediate strength (5 TU)
- Second strength (250 TU)
- Tuberculin test conversion is defined as an increase of 10 mm or more within a 2-year period, regardless of age.
Tuberculin test in use:
- Mantoux intradermal test: This is considered a more precise method for assessing tuberculin sensitivity.
- Heaf test: This test is valued for its speed, ease of use, reliability, and low cost, making it suitable for testing large groups.
- Tine multiple puncture test: This method is deemed unreliable and is not recommended for use.
- The effectiveness of the tuberculin test as an indicator of true infection prevalence has diminished in countries with high BCG vaccination coverage.
- True prevalence rates are artificially elevated due to infections with atypical mycobacteria and the boosting effect from a second dose of tuberculin.
Mantoux Test
- Dose: TU of PPD in 0.1 ml.
- Readings: Result read after 72 hrs (3 d).
- Dose: TU of PPD in 0.1 ml injected intradermally on the forearm.
- WHO recommended preparation: PPD-RT-23 with Tween-80.
- The Mantoux test is significant for prognosis.
- It has limited validity due to low specificity.
- Induration Measurements:
- Induration > 9 mm: Positive (indicates past or current TB infection).
- Induration 6-9 mm: Doubtful (possibly M. tuberculosis or atypical mycobacteria).
- Induration < 6> Negative.
- False Reactions:
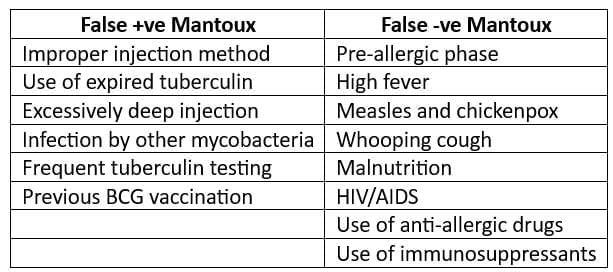
- Results of the tuberculin test must be interpreted carefully. The individual's medical risk factors determine at which increment (5 mm, 10 mm, or 15 mm) of induration the result is considered positive.
- 5 mm or more is positive in:
- HIV-positive individuals.
- Recent contacts of TB cases.
- Individuals with nodular or fibrotic changes on chest x-ray consistent with old healed TB.
- Patients with organ transplants.
- Other immunosuppressed patients.
- 10 mm or more is Positive in:
- Recent arrivals (less than 5 years) from high-prevalence countries.
- Injection drug users.
- Residents and employees of high-risk congregate settings (e.g., prisons, nursing homes, hospitals, homeless shelters).
- Mycobacteriology lab personnel.
- Individuals with clinical conditions that place them at high risk (e.g., diabetes, prolonged corticosteroid therapy, leukemia, end-stage renal disease, chronic malabsorption syndromes, low body weight).
- Children under 4 years, or children and adolescents exposed to adults in high-risk categories.
- 15 mm or more is Positive in:
- Individuals with no known risk factors for TB.
Sputum Microscopy & Culture
- Sputum smear examination (Z-N Staining) through direct microscopy is the preferred method for detecting tuberculosis.
- Sputum culture testing is available at district and regional chest clinic laboratories.
- This test is intended for individuals with chest symptoms who are smear-negative.
- It is useful for:
- Conducting sensitivity tests
- Monitoring drug treatment
Mass Miniature Radiography (MMR)
- This method is no longer utilized as a primary case-finding tool.
- However, it remains valuable for:
- Providing an supplementary criterion for diagnosing Pulmonary TB when two sputum smears yield negative results.
- Excluding conditions such as bronchiectasis or aspergilloma in patients with frequent or severe positive sputum smears.
- Identifying suspected complications in breathless patients requiring specific interventions, such as pneumothorax, pericardial effusion, or pleural effusion.
- Guidelines for Chemoprophylaxis in Children (< 6 years)
- (who come in contact with a sputum-positive TB case)
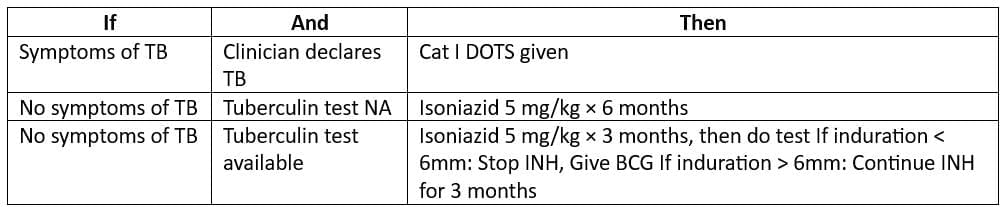
Stop TB Strategy
- Vision: A world free of TB
- Goal: To continue reducing the global burden of TB in line with the latest WHO objectives.
- Targets of strategy:
- 2005: Case detection rate > 70% and cure rate > 85%
- 2010: Reduce prevalence of & deaths by 50% (relative to 1990)
- 2015: Aim to eliminate TB as a public health problem, defined as less than 1 case per million population.
- Components:
- Pursue high quality DOTS expansion and enhancement
- Address TB/HIV, MDR TB and other challenges
- Contribute to health system strengthening
- Engage all care providers
- Empower people with TB and communities
- Enable and promote research
Latent Tuberculosis (LTBI)
- Description: Latent tuberculosis occurs when a person is infected with Mycobacterium tuberculosis, but does not have active tuberculosis disease.
- Latent TB is Not Infectious
- Main risk: There is a 10% chance that an individual will develop active TB later in life.
Tests for Identifying Latent TB:
- Tuberculin skin tests (Montoux test, Heaf test, Tine test)
- Alpha-interferon tests
Administering treatment for latent TB to a patient with active TB is a serious clinical mistake:
- TB will not be adequately treated
- There is a significant risk of developing drug-resistant strains of TB
Treatment Regimens for Latent TB:
- 6 months of Isoniazid
- 4 months of Rifampicin
- 3 months of Isoniazid + Rifampicin
- 4 months of Rifampicin +Pyrazinamide
Poliomyelitis
Situation in 2014 (Worldwide)
- Endemic Countries:
- Afghanistan
- Pakistan
- Nigeria
- Countries with Re-established Transmission:
- Cameroon
- Somalia
- Syria
- Ethiopia
- Kenya
- Chad
Situation in 2013 (India)
- Total Cases: No wild virus cases reported since January 13, 2011.
- VDPV Cases:. cases of Vaccine-Derived Poliovirus (P2) reported.
Poliomyelitis Disease
- Causative Agent: Poliovirus (Serotypes 1, 2, and 3)
- Major Cause of Epidemics: Poliovirus, particularly serotype 1, is the primary driver of polio outbreaks.
- Antigenic and Eradication Potential: Serotype 1 is the most antigenic and easiest to eradicate, making it a key target for vaccination efforts.
- Poliovirus Type 3 (P3): P3 is significant because it is the main cause of Vaccine Associated Paralytic Poliomyelitis (VAPP), a rare but serious complication of vaccination.
- VAPP Risk: The risk of VAPP is approximately 1 in 1 million vaccine doses, highlighting the safety of the vaccine despite this rare adverse effect.
- Reservoir: Humans are the only reservoir for poliovirus, and there are no chronic carriers of the virus.
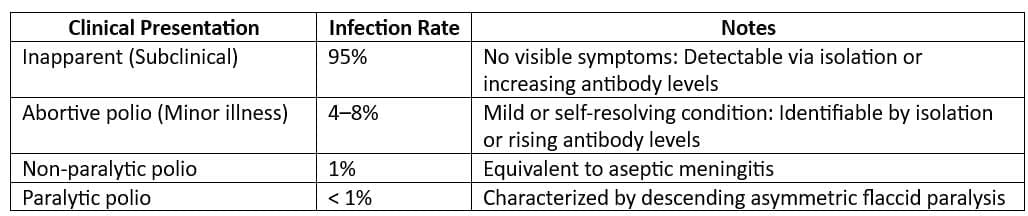
Most Common Clinical Occurrence: Subclinical cases of poliomyelitis are the most common, with a ratio of approximately 1,000 subclinical cases in children and 75 subclinical cases in adults for every 1 clinical case of polio. - Infectious Material: Poliovirus is present in faeces and oro-pharyngeal secretions of infected individuals.
- Period of Communicability: Infected individuals can spread the virus 7-10 days before and after the onset of symptoms.
- Risk Factors for an Attack:
- Fatigue
- Trauma
- Intramuscular Injections
- Operative Procedures: Tonsillectomy and similar procedures are particularly risky during polio epidemics.
- Administration of Alum-Containing DPT Vaccine
- Incubation Period: The incubation period for poliomyelitis ranges from 3 to 35 days, with a usual duration of 7 to 14 days.
Diagnostic Tests for Poliomyelitis
- Stool Examination:
- The isolation of wild poliovirus from stool samples is the preferred method for confirming paralytic poliomyelitis in the laboratory.
- This test is recommended for all cases of acute flaccid paralysis (AFP).
- The virus can usually be detected in feces from the onset of symptoms up to 8 weeks after the onset of paralysis, with the highest likelihood of detection during the first 2 weeks after paralysis begins.
- Cerebrospinal Fluid (CSF) Examination:
- CSF examination is not recommended for surveillance purposes in cases of poliomyelitis.
- It is unlikely to yield the virus, so collecting CSF for culture is not advised.
- However, results such as cell count, gram stain, protein, and glucose levels in the CSF may be helpful in ruling out other conditions that cause acute flaccid paralysis.
- Throat Examination:
- Throat examinations are less likely to yield the virus compared to stool samples, so collecting samples from this site is not recommended for poliovirus detection.
- Blood Examination:
- Blood examination is also unlikely to yield the virus, and current serologic tests cannot differentiate between wild and vaccine-derived poliovirus strains.
- Interpreting serologic data can often be misleading, so collecting blood specimens for culture or serology is not recommended in the context of poliomyelitis.
- Vaccines for Poliomyelitis:
- For information on vaccines for poliomyelitis, please refer to Chapter 3 Theory.
Hepatitis
Types of Viral Hepatitis
Hepatitis A
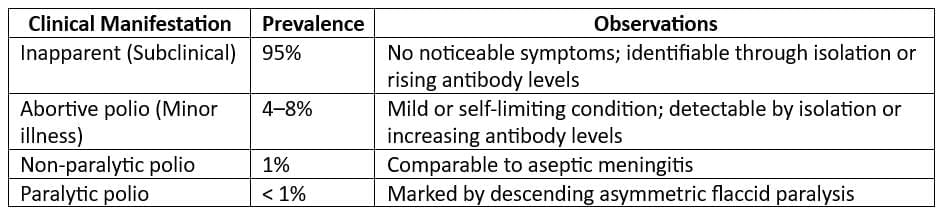
- Incubation period: 15 - 45 days
- Period of infectivity: weeks before to 1 week after onset of jaundice
- Modes of transmission: Faecal-oral (most common)
- Gender distribution: Balanced across both genders
- Children: Higher infection rates but typically mild or subclinical
- Reservoir: Human cases
- Transmission methods:
- Faecal-oral (Most frequent)
- Parenteral
- Sexual
- Disinfectant options:
- Formalin
- UV rays
- Boiling for 5 minutes
- Autoclaving
Hepatitis B
- Causative agent: Hepatitis B virus (HBV) – a Hepadnavirus
Is also known as: Dane particle or double-shelled DNA virus
Reservoir of infection: Humans (case or carrier)
- Causative agent: Hepatitis B virus (HBV) – a Hepadnavirus
Incubation period: 45–180 days (median 60–90 days or 6 weeks–6 months)
Modes of transmission: Bloodborne, sexual, parenteral; perinatal
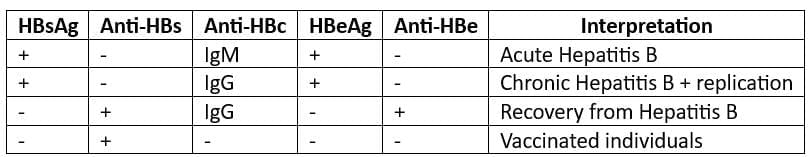
Markers of Hepatitis B infection (in order of appearance in serum):
HBsAg (Hepatitis B surface antigen):
- Also known as Australia antigen
- First antigen to appear in serum – first evidence of infection
Epidemiological marker of Hepatitis B infection
HBeAg (Hepatitis Be antigen):
Alone does not appear in serum
HBeAg (Hepatitis B envelope antigen):
- Is a secretory form of HBeAg
- Indicates active viral replication
- Is a marker of infectivity for Hepatitis B
- Resistance beyond 3 months: Increased likelihood of chronic Hepatitis B
- Anti-HBc (Antibody to Hepatitis B core antigen):
- First antibody to appear in serum
- IgM Anti-HBc indicates a diagnosis of acute Hepatitis B
- IgG Anti-HBc persists indefinitely
- Anti-HBe (Antibody to Hepatitis B e envelope antigen):
- Signals “stoppage of active viral replication”
- Indicates end of period of infectivity
- Anti-HBs (Antibody to Hepatitis B surface antigen):
- Last antibody to appear in serum
- Signals recovery, end of period of communicability
- Serologic patterns in Hepatitis B:
- Vaccines for Hepatitis B:
- Plasma-derived vaccine
- Is formalin-inactivated sub-unit vaccine
- Is based on HBsAg
- Derived from carriers of Hepatitis B
- rDNA yeast-derived vaccine
- Recombinant DNA vaccine (genetically engineered)
- Hepatitis B Immunoglobulin:
- Needed for immediate protection
- Surgeons, nurses, laboratory workers
- Newborn infants of carrier mothers
- Sexual contacts of acute Hepatitis B patients
- Ideally administered within 6 hours (not later than 48 hours)
- Dose: 0.05–0.07 ml/kg, 2 doses 30 days apart
Hepatitis E
- Synonym: Enterically transmitted hepatitis non-A, non-B (HNANB)
- Description: HEV is mainly a waterborne disease, transmitted through water or food supplies contaminated by faeces
- Incubation Period: 2- 9 weeks
- HEV in pregnancy: Fulminant form is common in Hepatitis E infection during pregnancy (up to 20% of cases) with a variable case fatality rate (up to 80%)
Diarrhoeal Diseases (Cholera & Typhoid)
Oral Rehydration Solution (ORS)
- ReSoMal (Rehydration Solution for Malnourished): Recommended for severely malnourished children
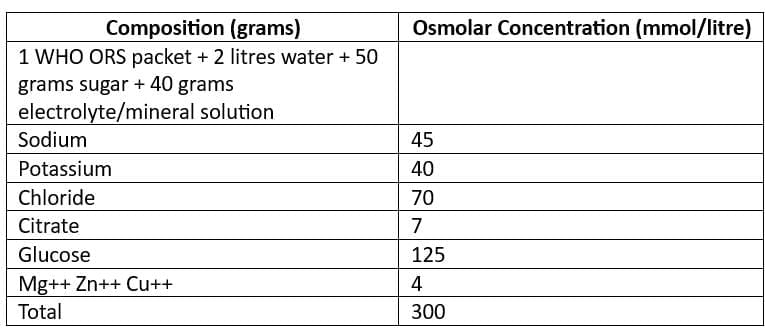
- New WHO Recommended Reduced Osmolarity Oral Rehydration Solution (Low Na ORS)
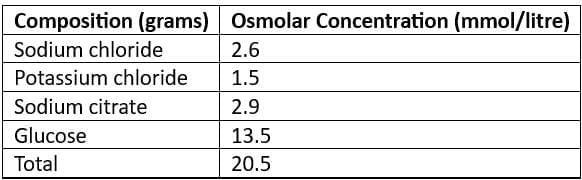
Cholera
- Cholera: An acute diarrhoeal disease caused by Vibrio cholerae
- Vibrio cholerae: Gram-negative bacterium producing cholera toxin (enterotoxin), affecting the c-AMP system of mucosal cells in the small intestine (causing severe diarrhoeal)
- Biotypes: Classical biotype, El Tor biotype [Serotypes: Ogawa (Most common in India), Inaba and Hikojima]
- Recent trends: El Tor Hybrid subtype has become most common in India
- Cholera: Characterized by Rice-Watery Diarrhea
- Cholera is a disease that is required to be reported to health authorities.
- Mass chemoprophylaxis is not recommended for cholera.
- The most effective prevention of cholera is through health education.
- Incubation Period:
- The incubation period for cholera typically ranges from 1 to 2 days, but it can vary from a few hours to as long as 5 days.
- Reservoir:
- Humans are the primary reservoir for cholera.
- Environmental sources may also contribute to the transmission of the disease.
- Rice-Watery Diarrhea
- The essential treatment for cholera includes:
- Water and electrolyte replacement, such as Oral Rehydration Solution (ORS)
- Laboratory Diagnosis of Cholera
- The most useful specimens for diagnosing cholera are:
- Stool and swab samples collected during the acute phase of the disease.
- These samples should be taken before the administration of antibiotics.
- Holding or Transport Media:
- Venkataraman-Ramakrishnan (VR) medium
- Cary-Blair medium
- Autoclaved sea water
- Enrichment Media:
- Alkaline peptone water
- Monsur's taurocholate tellurite peptone water
- Plating Media:
- Alkaline bile salt agar (BSA)
- Monsur's gelatin taurocholate trypticase tellurite agar (GTTA) medium
- Thiosulfate-citrate-bile salts-sucrose (TCBS) medium, which is the most commonly used medium for cholera diagnosis
Guidelines for Cholera Control (WHO)
- Verification of Diagnosis:
- Finding Vibrio cholerae 01 in the stools of a few patients is sufficient for diagnosis.
- It is not necessary to culture stools from all cases or contacts.
- Notification:
- Cholera is a notifiable disease at local, national, and international levels.
- According to International Health Regulations, it must be reported to WHO by national governments within 24 hours, including the number of cases and deaths daily and weekly.
- An area is considered free of cholera when twice the incubation period has passed since the last case.
- Early Case Finding:
- Conduct proactive case identification.
- Establish treatment centres.
- Use rehydration therapy with ORS (Oral Rehydration Solution).
- Administer antibiotics only when vomiting has stopped.
- Epidemiological Investigations:
- Implement general sanitation measures and conduct epidemiological studies to understand the outbreak.
- Sanitation measures include:
- Water control to ensure safe drinking water.
- Proper excreta disposal to prevent contamination.
- Food sanitation to ensure safe food handling and preparation.
- Disinfection of contaminated surfaces and materials.
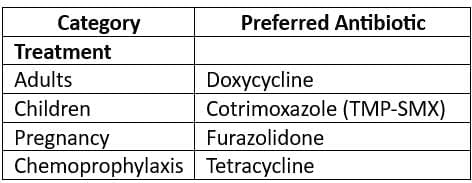
- Chemoprophylaxis:
- Mass chemoprophylaxis is not recommended for the entire community; it is only advised for household contacts or closed communities at risk.
- The drug of choice for chemoprophylaxis is Tetracycline.
- It is estimated that 10,000 individuals must receive chemoprophylaxis to prevent one case of cholera.
- Vaccination:
- Health education is the most effective preventive measure against cholera.
- Causative agent:Vibrio cholerae is the bacterium responsible for cholera.
- Reservoir of infection: Humans, including both cases and carriers, are the primary reservoirs of infection.
- Incubatory carriers and convalescent carriers can excrete the bacilli for 6-8 weeks after infection.
- Chronic carriers have the ability to excrete bacilli for more than 1 year following the clinical attack.
- Source of infection: The primary sources of infection include feces and urine from cases and carriers. Secondary sources include contaminated water, food, fingers, or flies.
- Incubation period: The incubation period for cholera is typically 10-14 days.
- Mode of transmission: Cholera is transmitted through the fecal-oral and urine-oral routes.
Clinical Features:
- The hallmark of cholera is "pea soup diarrhoea," which is characterized by watery stools with a consistency resembling pea soup.
- Other signs that may be observed in cholera patients include:
- Splenomegaly: Enlargement of the spleen.
- Relative bradycardia: Slower than normal heart rate.
- Dicrotic pulse: Characteristic pulse wave pattern.
- Abdominal distension and tenderness: Swelling and sensitivity in the abdominal area.
- Rose spots: These are small, rose-colored spots that may appear on the skin during the second week of infection.
- Intestinal perforation: This serious complication may occur in the third week of infection.
Laboratory Diagnosis:
- Use the “BASU” mnemonic for laboratory diagnosis.
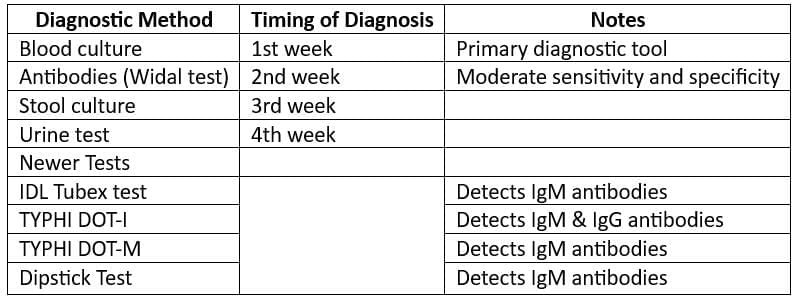
- Drug of Choice:
- For cases of cholera: Cephalosporins (such as Ceftriaxone) and Quinolones are the preferred antibiotics.
- For carriers of cholera: Ampicillin or Amoxicillin combined with Probenecid for 6 weeks is recommended.
- Immunisation for Typhoid:
- TYPHORAL (Live oral Ty21a) vaccine:
- This vaccine contains more than 10 viable organisms of attenuated S. typhi.
- Schedule: One capsule each on days 1, 3, and 5, with a booster of 3 doses every 3 years.
- Protection duration: 3 years.
- TYPHIM Vi Vaccine:
- This Vi-Polysaccharide vaccine is given as a single dose either intramuscularly or subcutaneously.
- This vaccine is not given to individuals under 2 years of age.
- TAB vaccine:
- This vaccine contains S. typhi, S. paratyphi A, and S. paratyphi B and provides immunity against these strains.
- TYPHORAL (Live oral Ty21a) vaccine:
Worm Infestations
Guinea Worm Infestations
- Causative agent: Dracunculus medinensis (a type of nematode)
- Guinea worm disease in India:
- Last reported case in India: July 1996 (Jodhpur, Rajasthan)
- India certified for elimination of Guinea worm by WHO: February 2000
- India declared Guinea worm disease-free: February 2001. Ongoing surveillance is essential to maintain this status.
- Type of biological transmission: Cyclo-developmental transmission
- Reservoir of infection: An infected person (there is no animal reservoir)
- Type of disease: Water-based disease (Cyclops play a role in transmission)
- Mode of transmission: Ingestion of water containing Cyclops that harbour the infective stage of the parasite
- Guinea worm is amenable to eradication:
- Provision of safe drinking water
- Implementing control measures for Cyclops populations
- Active surveillance for cases
- Treatment of cases: Niridazole, Mebendazole, and Metronidazole
- Prevention: There is no effective drug for preventing disease transmission, and no drug is suitable for mass treatment.
Roundworm (Ascariasis)
- Importance:
- It is the most prevalent helminthic infection.
- It is the most common worm infestation in India.
- Causative agent: Ascaris lumbricoides
- Reservoir of infection: Humans
- Mode of transmission: Faecal-oral route
- Incubation period: Approximately 2 months
- Drugs for treatment:
- Albendazole
- Pyrantel
Hookworm (Ancylostomiasis)
- Causative agent:'
- Ancylostoma duodenale
- Mode of transmission: Direct penetration of skin (usually through the foot) and by oral route
- Incubation period:
- 4-9 weeks for A. duodenale
- 6-8 weeks for Necator americanus
- Hookworm infection is also known as: miners' anaemia, tunnel disease, brickmaker's anaemia, Egyptian chlorosis
- Average blood loss in hookworm infection: 0.03-0.2 ml per worm per day
- Associated conditions:
- Iron deficiency anaemia
- Hypoalbuminaemia
- Cutaneous larva migrans:. skin disease in humans caused by the larvae of various nematode parasites, most commonly Ancylostoma braziliense
- Drugs of choice for treatment:
- Albendazole for A. duodenale
- Mebendazole for N. americanus
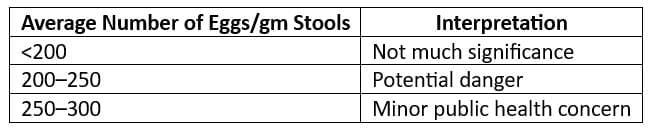
Endemic Index (Chandler's Index):
- CI is the average number of hookworm eggs per gram of faeces for the entire community.
- Interpretation of CI: Kato-Katz Technique is employed.
Tapeworm (Taeniasis)
Causative agent:
- Taenia solium
- Taenia saginata

Dengue & Yellow Fever
Dengue Fever and Related Syndromes
- Causative Agent: Dengue viruses, classified as arboviruses.
- Serotypes: There are four serotypes of dengue virus: Den 1, 2, 3, and 4.
- Vector: The primary vector for dengue transmission is the Aedes aegypti mosquito.
- Reservoir: Humans and mosquitoes serve as reservoirs for the dengue virus.
- Incubation Period: The incubation period for dengue is typically 5-6 days.
- Classical Dengue Fever (DF):
- Also known as "breakbone fever," DF is characterized by the following clinical features:
- High-grade fever with a biphasic curve and accompanying chills.
- Intense headache.
- Muscle and joint pains.
- Retro-orbital pain (pain behind the eyes).
- Photophobia (sensitivity to light).
- Colicky abdominal pain.
- Abdominal tenderness.
- Skin rash.
- Dengue Hemorrhagic Fever (DHF):
- DHF is a severe form of DF that occurs when a person is infected with more than one type of dengue virus.
- Incubation Period: 4-6 days
- Clinical Features: In addition to the features of DF, DHF includes:
- Rash is less common in DHF.
- Rising hematocrit value (> 20% of baseline).
- Moderate-to-marked thrombocytopenia (< 1000/sq.mm)
- Positive tourniquet test: More than 20 petechiae per square inch.
- Diagnosis of DHF: Requires the presence of fever, hemorrhagic manifestations, thrombocytopenia, and hemoconcentration or rising hematocrit.
- Dengue Shock Syndrome (DSS):
- DSS is diagnosed when DHF is accompanied by shock, characterized by a rapid and weak pulse, narrow pulse pressure (< 20 mm hg) hypotension, cold, clammy skin.
Yellow Fever (Also known as Yellow Jack, Black Vomit, or American Plague)
- Causative agent:Flavivirus fibricus (Togavirus Family, Group B Arbovirus)Reservoir of infection:
- Forest (Sylvian) Cycle: Monkeys and forest mosquitoes
- Urban Cycle: Humans (subclinical and clinical cases) and Aedes aegypti
- Period of communicability:
- Humans: First 3–4 days of illness
- Mosquitoes: Lifelong (after extrinsic incubation period of 8–12 days)
- Immunity:Single infection provides lifelong immunity
- Infants born to immune mothers have antibodies up to 6 months of life
- Incubation period:3–6 days
- Recognized under International Health Regulations
- Case fatality rate: 80%
- Yellow Fever Vaccine:
- Live attenuated, lyophilized (freeze-dried) vaccine
- Strain: 17D strain (Chick Embryo grown)
- Cold chain temperature: -30° to +5°C
- Reconstitution with Diluent: Cold physiological saline
- After reconstitution, use within ½ hour
- Dose: 0.5 ml (irrespective of age)
- Route: Subcutaneous
- Site: At insertion of Deltoid
- Immunity lasts: From 7 days of vaccination till 35 years
- WHO recommended validity of Vaccination Certificate for international travel: From 10 days to 10 years
- YF vaccine is the only live vaccine that can be administered in pregnancy (if there is risk of exposure)
- Yellow Fever Vaccine and Cholera Vaccine cannot be given together: Maintain a gap of 3 weeks or more between them
- Indices of Surveillance of Aedes Mosquitoes
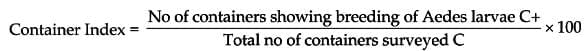


- Yellow Fever Control Measures:
- Distance around airports: Maintain a 400-meter radius free of Aedes breeding sites
- Breteau Index (Aedes aegypti index): Should be less than 1% in towns and seaports
|
30 docs|6 tests
|
FAQs on Communicable and Non-communicable Diseases - 1 Chapter Notes - Preventive and Social Medicine (PSM) - NEET PG
| 1. What are communicable diseases and how do they differ from non-communicable diseases? |  |
| 2. What is the period of communicability for common communicable diseases? |  |
| 3. What are the common gestational periods for vertical transmission of diseases? |  |
| 4. What are the incubation periods for some common diseases? |  |
| 5. Can you explain the significance of important human parasites and their hosts? |  |















