NCERT Based Activity: Particulate Nature of Matter | Science Curiosity Class 8 - New NCERT PDF Download
Activity 7.1: Let us explore
- Take a stick of chalk (Fig. 7.1a) and break it into two pieces (Fig. 7.1b).

- Continue breaking the chalk till it becomes difficult to break it further by hand.
- Grind the small pieces of chalk thus obtained (Fig. 7.1c) using mortar and pestle.
- Observe the fine powder of chalk with a magnifying glass (Fig. 7.1d).
- What do you observe?
- Each tiny grain you observe is still a speck of chalk.
- Is every speck of this fine chalk powder still composed of the same substance, or has it changed into something else on breaking or grinding?
Answer: Each tiny grain observed is still a speck of chalk, composed of the same substance. Breaking or grinding does not change it into something else; it is a physical change that only reduces the size of each speck.
Activity 7.2: Let us perform
- Fill a glass tumbler with drinking water.
- Put two teaspoons of sugar into it.
- Do not stir the water. Taste a small spoonful of water from the top layer of the glass.
- Does the water taste sweet?
- Now, stir the water until the sugar dissolves completely (Fig. 7.2).

- Again taste a spoonful of water from the top layer.
- What difference in taste do you notice? Does it taste sweet?
- Since the top layer of water tastes sweet after dissolving sugar, it must be present in the solution. Do you observe any sugar particles in the solution?
Answer: Initially, the water does not taste sweet. After stirring and dissolving the sugar completely, the water tastes sweet. The taste difference is due to the sugar dissolving. No sugar particles are observed in the solution, but their presence is sensed by taste. When sugar dissolves, it breaks into constituent particles which cannot be broken down further.
Activity 7.3: Let us find out
- Collect a few solid objects, such as a piece of iron or an iron nail, a piece of rock salt, a stone, a piece of wood, a key, and a piece of aluminium (Fig. 7.3).

- Observe their shapes and sizes.
- Try hammering them.
- In which of the above six objects do you think particles are strongly held together?
- In the solid state, is there any way to move these particles apart?
Answer:
- The particles are strongly held together in objects like the iron nail, rock salt, stone, and piece of aluminium, as they resist hammering and maintain their shape.
- The wooden block and key may deform slightly but are also relatively strong. In the solid state, particles can be moved apart by heating, which increases their vibrations until they overcome interparticle forces, converting the solid into a liquid at its melting point.
Activity 7.4: Let us try and find out
- Take three clean and dry containers of different shapes. Label them A, B, and C (Fig. 7.5).
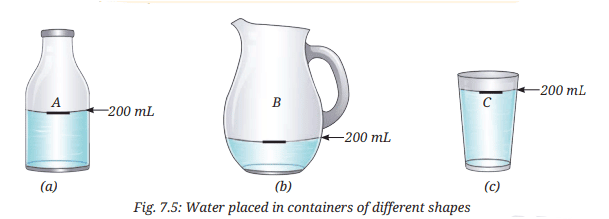
- Mark the 200 mL level in each container using a marker or by pasting a thin strip of paper.
- Fill Container A with water up to the marked level.
- Carefully transfer the water from Container A to Container B without spilling, and observe the shape and level of the water.
- Now, transfer the same water from Container B to Container C, carefully, and observe the shape and its level again.
- Are you able to move your finger through the water?
Answer:
- The water takes the shape of each container (A, B, and C) but maintains a level of 200 mL, indicating it has a definite volume but no fixed shape.
- Yes, you can move your finger through the water without permanently breaking it, as the particles are free to move within a limited space, and the position is restored when the finger is removed.
Activity 7.5: Let us investigate
- Take two transparent gas jars or glass tumblers and mark them A and B.
- Create some smoke by burning an incense stick.
- Hold the Gas Jar A upside down over the smoke (Fig. 7.7a).
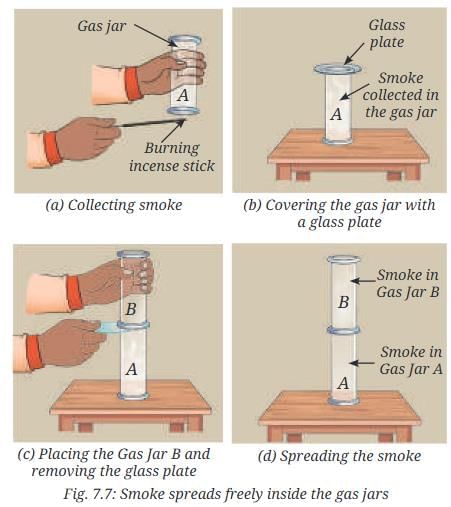
- The gas jar should trap the smoke inside.
- Turn it over and cover it with a glass plate (Fig. 7.7b).
- Hold another Gas Jar B upside down and gently place it over the glass plate covering the Gas Jar A.
- Remove the glass plate slowly and ensure that both gas jars are close enough and there is no gap for smoke to escape (Fig. 7.7c).
- Observe how the smoke spreads inside the Gas Jar B.
- Do gases also have a fixed volume?
Answer: The smoke spreads and fills the entire space in Gas Jar B, indicating that gases do not have a fixed volume and acquire the shape of the vessel. This shows that gas particles move freely in all directions with negligible interparticle attractions.
Activity 7.6: Let us experiment
- Take a syringe without a needle. Pull the plunger of the syringe outwards in a fully extended position (Fig. 7.9a).
- Place your thumb over the open end of the syringe to prevent the air present inside the syringe from escaping (Fig. 7.9b).
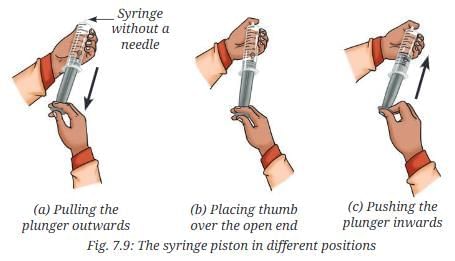
- Push the plunger slowly and steadily inward (Fig. 7.9c).
- What do you observe?
- What can we say about the behaviour of gas in the syringe?
Answer: The volume of air inside the syringe decreases as the plunger is pushed. This shows that gas particles have a lot of space between them, which can be reduced by applying external pressure. When the plunger is stopped, the gas particles spread, moving the plunger back.
Activity 7.7: Let us observe
- Take a glass vessel, fill it about half with water, and mark the level of water A (Fig. 7.10a).
- Add two teaspoons of sugar into it.
- Mark the new water level on the glass vessel B (Fig. 7.10b).
- Stir the water with a glass rod to dissolve the sugar (Fig. 7.10c).
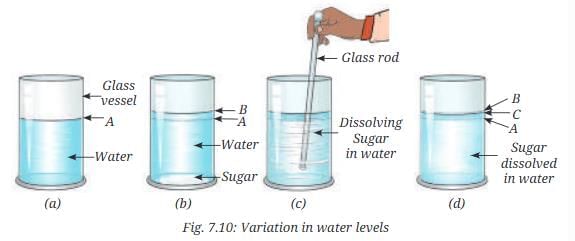
- Predict whether the water level will increase or decrease with respect to the mark B.
- Mark this water level again as C (Fig. 7.10d).
- What difference do you observe in the water levels?
- Repeat the Activity 7.7 with some other soluble solids, such as common salt or glucose, and insoluble solids, like sand and stone pieces.
- What do you observe in each case? Do the sand particles dissolve? Does the volume of water in the vessel change after mixing, and why?
- Sugar and sand are both solids. Why does sugar dissolve in water but sand does not?
Answer:
- Initially, the water level increases from A to B when sugar is added. After dissolution (level C), it may decrease slightly below B, indicating that sugar particles occupy interparticle spaces.
- With soluble solids like salt or glucose, the level behaves similarly. With insoluble solids like sand or stone, they settle and increase the total volume without dissolving.
- Sand does not dissolve because its particles are held together strongly and cannot be separated by water, unlike sugar’s constituent particles.
Activity 7.8: Let us experiment
- Take a glass tumbler containing water and put a few grains of potassium permanganate into it.
- What do you observe?
- Initially, you will see some streaks of pink colour spreading out from the grain (Fig. 7.13a).
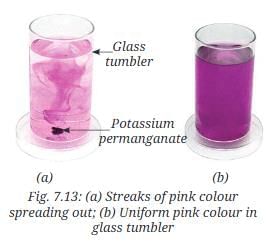
- With the passage of time, the entire bulk of water will acquire a uniform pink colour (Fig. 7.13b).
- Do you know why this happens?
- Try it yourself! Take three clean glass tumblers. Pour hot water in one of them, water at room temperature in the second, and ice-cold water in the third. Drop a small grain of potassium permanganate into each of them. Watch carefully and compare. What do you observe?
- How can we demonstrate the movement of gas particles that cannot be seen with the naked eye?
Answer:
- Streaks of pink colour spread out initially, and eventually, the water turns uniformly pink because water particles are in constant motion, pulling and spreading potassium permanganate particles.
- In hot water, the colour spreads fastest, slower in room temperature water, and slowest in ice-cold water, as particle movement increases with heat.
- The movement of gas particles can be demonstrated using Activity 7.9 with an incense stick.
Activity 7.9: Let us find out
- Light an incense stick in one corner of the room (Fig. 7.14).

- Wait for a few minutes and observe.
- Do you notice the fragrance from a distance?
- Can you share a few other real-life situations where you have experienced the movement of gas particles?
Answer: The fragrance is initially felt around the incense stick and soon spreads throughout the room, showing that air particles move constantly and help spread the fragrance. Other situations include the spread of cooking smells, perfume, or car exhaust fumes.
|
59 videos|235 docs|13 tests
|
FAQs on NCERT Based Activity: Particulate Nature of Matter - Science Curiosity Class 8 - New NCERT
| 1. What is the particulate nature of matter? |  |
| 2. How do scientists demonstrate the particulate nature of matter? |  |
| 3. What are the different states of matter and how do they relate to the particulate nature of matter? |  |
| 4. Why is the study of the particulate nature of matter important in science? |  |
| 5. What role did historical figures play in developing the concept of the particulate nature of matter? |  |





















