Cardiology Chapter Notes | Medicine - NEET PG PDF Download
Introduction to ECG: Understanding Tachyarrhythmia and Bradyarrhythmia
P Wave
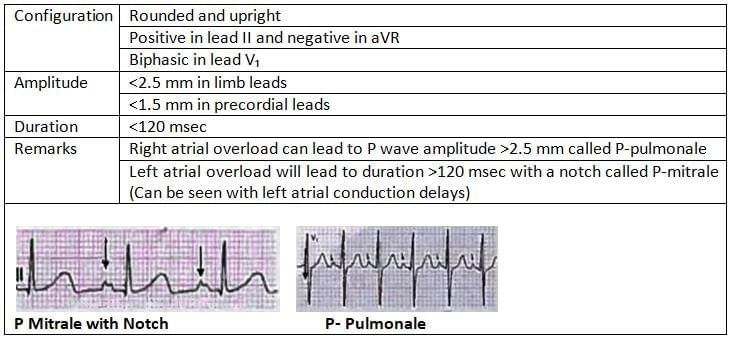 Pseudo P- Pulmonale is seen in Hypokalemia
Pseudo P- Pulmonale is seen in Hypokalemia
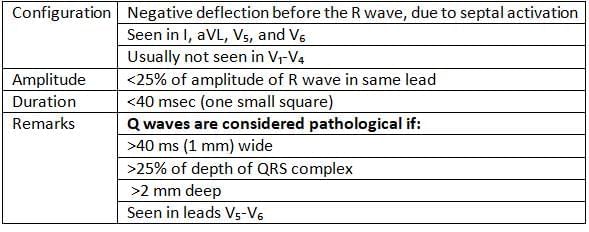
qWave
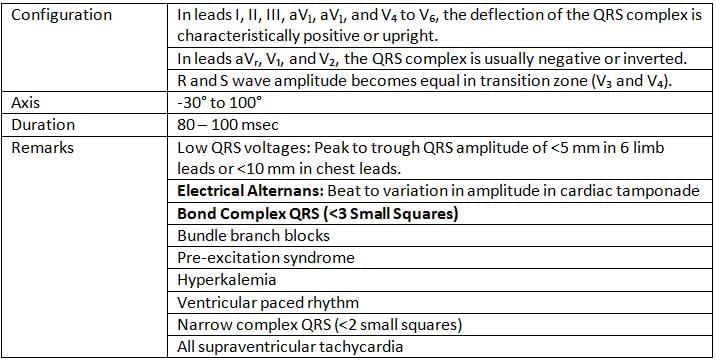
QRS Complex
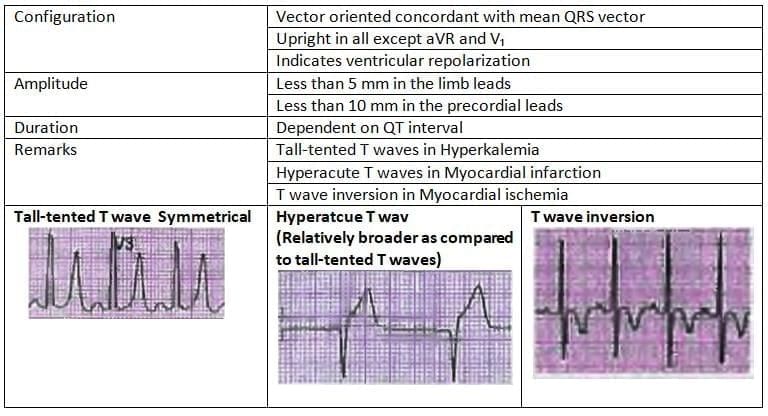
T Wave
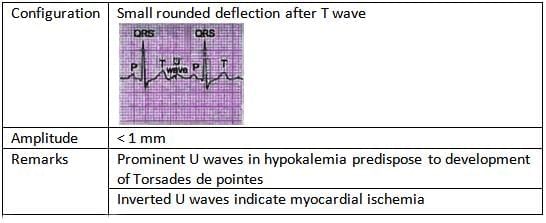
U Wave
PR Interval

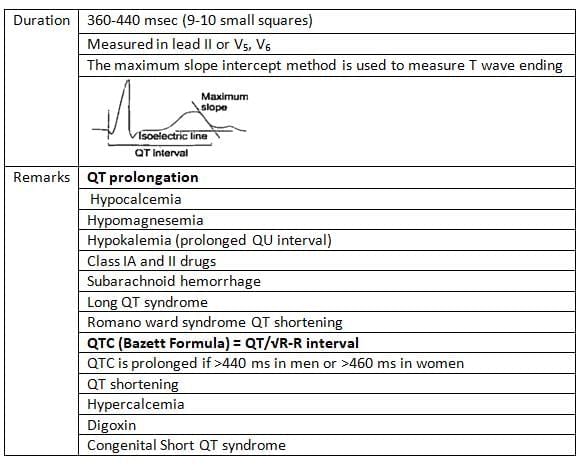
QT Interval
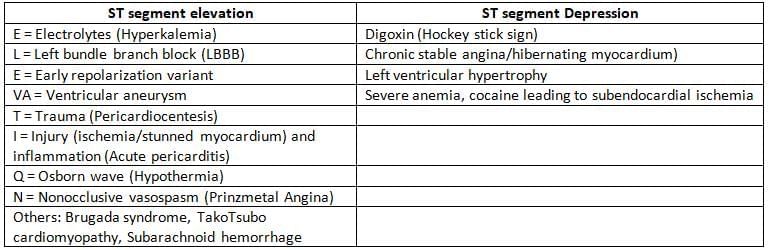
ST Segment Changes
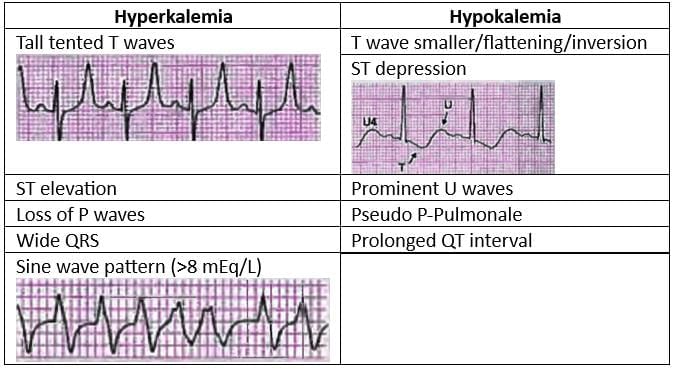
Electrolytes and ECG Abnormalities

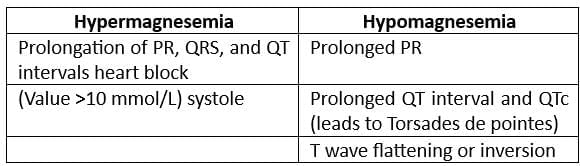
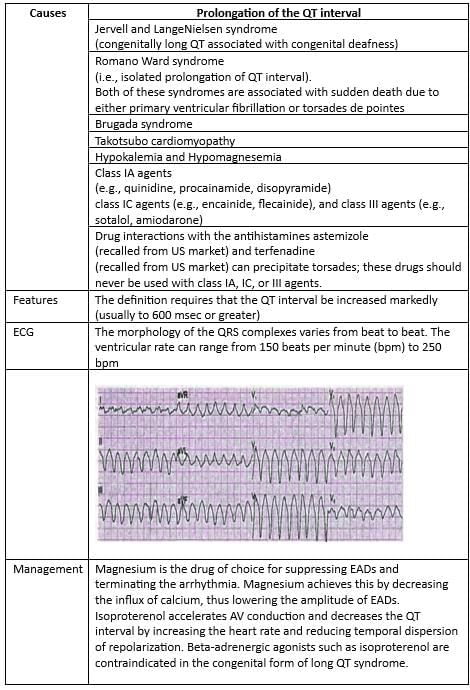
QT Prolongation is seen with both Hypermagnesemia and Hypomagnesemia
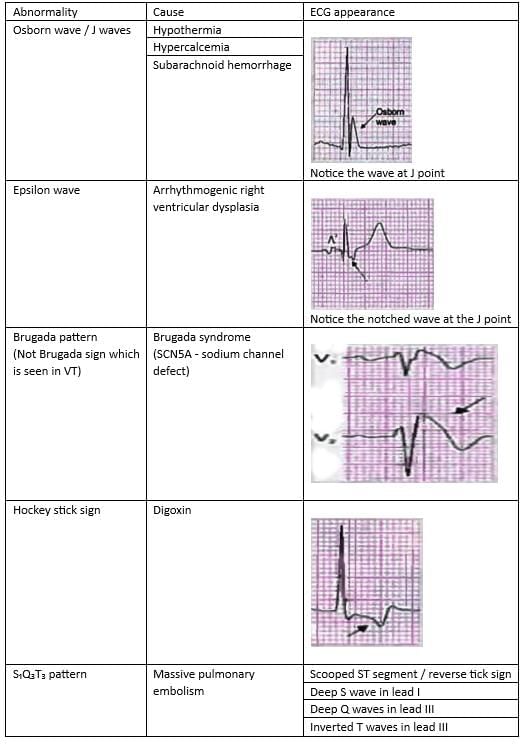
Miscellaneous Aberrations
 Hockey stick sign on echocardiography is seen in mitral stenosis
Hockey stick sign on echocardiography is seen in mitral stenosis
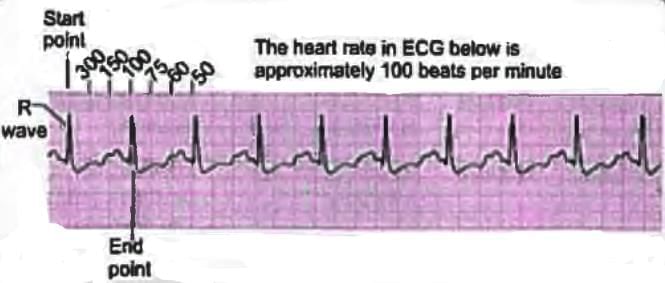
Heart Rate Calculation
- Method 1: Count the number of large squares between the R-R intervals.
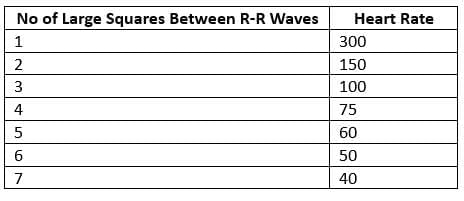
- Method 2: Divide 1500 by the number of small squares counted between the R-R interval.
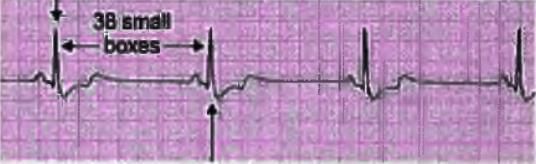
- Method 3: Heart rate calculation in abnormal rhythm
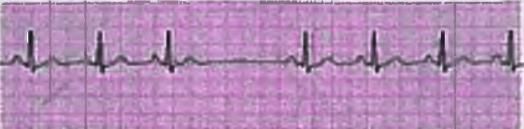
- In cases where the R-R interval is variable, count the number of R waves in a 6-second strip and multiply by 10.
- For instance, if there are 7 R waves in the 6-second interval of the strip, the heart rate would be 7 x 10 = 70 beats per minute.
Axis Calculation
- The most effective way to calculate the heart axis is by determining the mean QRS vector from lead I and aVF.
- These vectors can be plotted on a diagram to accurately obtain the heart axis.
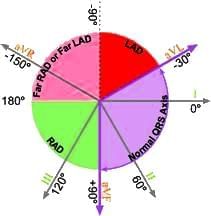
Interpretation
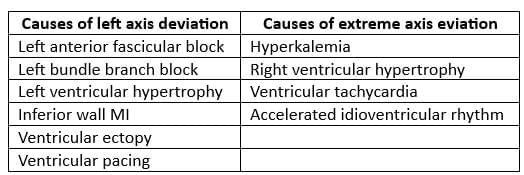
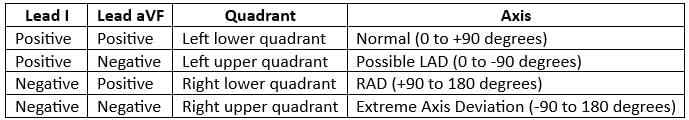 Right Axis Deviation
Right Axis Deviation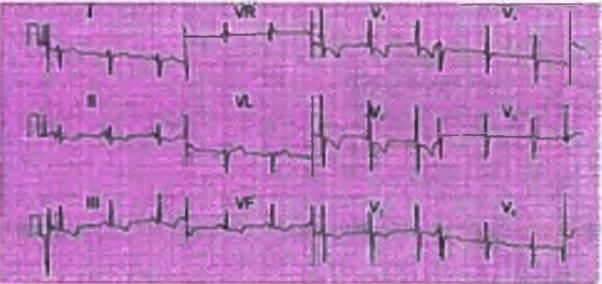
- The Mean QRS vector in lead 1 is negative
- The Mean QRS vector in lead aVF is positive
- Therefore, the axis indicates right axis deviation
Causes of Right Axis Deviation
- Normal (Children and young adults)
- Left posterior fascicular block
- Reversal of right and left arm electrodes
- Pulmonary embolism
- Pulmonary arterial hypertension (PAH)
- Chronic obstructive pulmonary disease (COPD)
- Lateral wall myocardial infarction (MI)
- Dextrocardia
- Left pneumothorax
- Wolff Parkinson White (WPW) syndrome
Left Axis Deviation
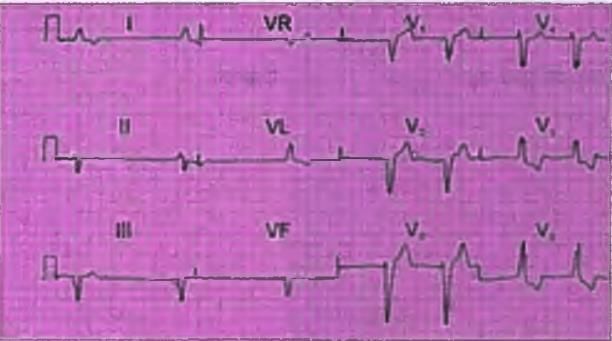
- The Mean QRS vector in lead I is Positive
- The Mean QRS vector in lead III is Negative
- This indicates that the axis shows a left axis deviation
To estimate the axis accurately, leads I and aVF are checked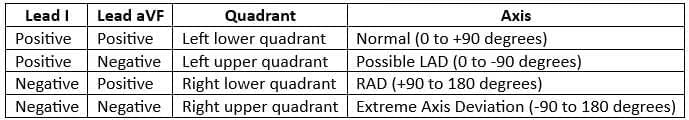
Bundle Branch Block
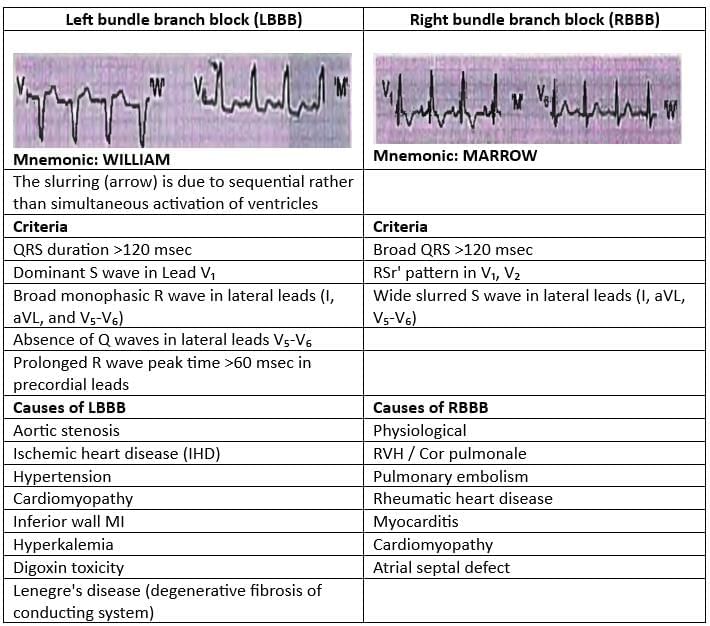
LBBB
- Dominant S wave in Lead V1
- Broad monophasic R wave in lateral leads (I, a VL, V5 - V6)
- Absence of Q waves in lateral leads V5 - V6
- Slurring of peak of R wave in V5 - V6
RBBB
- Notice the RSR' pattern in V1 - V3
- Wide slurred S wave in lateral leads (I, a VL, V5 - V6)
Chamber Enlargement
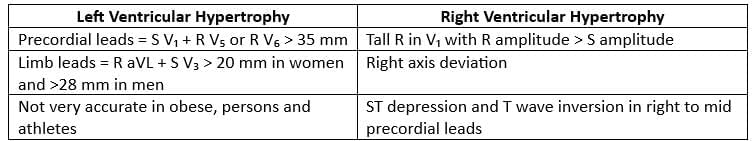
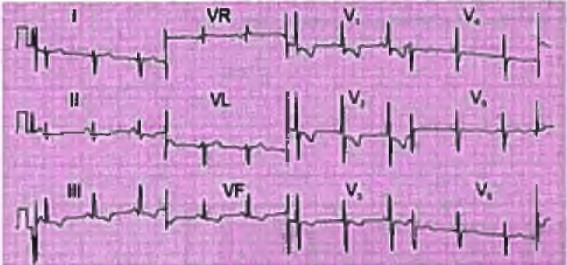
Left Ventricular Enlargement
- The ECG indicates a normal sinus rhythm with a heart rate of 50 beats per minute (bpm).
- The QRS axis is normal.
- R wave height in lead V5 is 30 mm.
- S wave depth in lead V1 is 20 mm.
- The sum of the R and S wave heights exceeds the criteria for left ventricular hypertrophy (LVH).
- Inverted T waves are observed in leads I, aVL, and V5 - V.
- Echocardiography is necessary to confirm the cause of left ventricular enlargement.
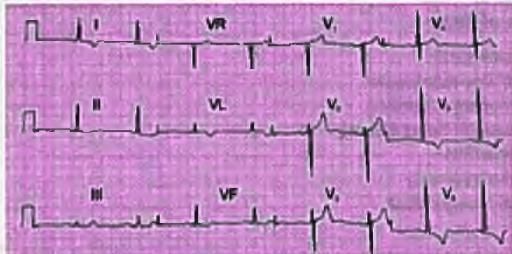
Right Chamber Enlargement
- The ECG shows a normal sinus rhythm with a heart rate of 75 bpm.
- There is right axis deviation with peaked P waves in leads II and III.
- T waves are inverted in leads II, III, aVF, and V1-V6.
- These findings suggest right ventricular strain possibly due to pulmonary artery hypertension.
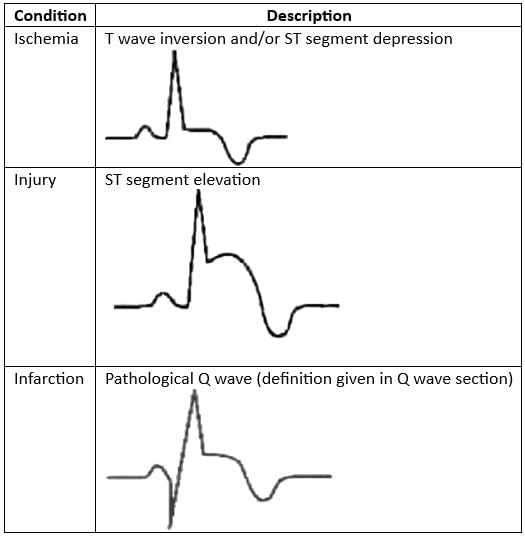
ECG Indicators of Myocardial Ischemia/Infarction
Infarct Localization
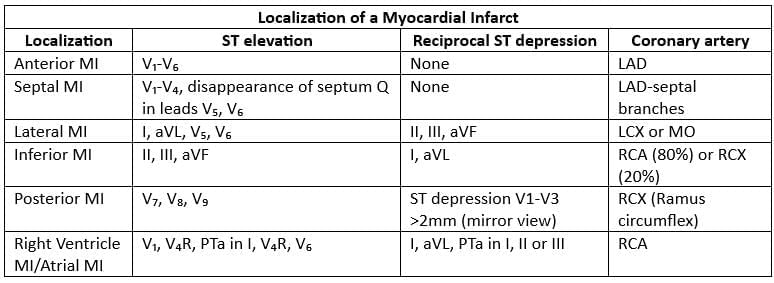
- LAD = left anterior descending artery
- LCX = left circumflex artery
- MO = marginal obtuse branch
- RCX = ramus circumflex
Quick Overview of Rhythm Abnormalities
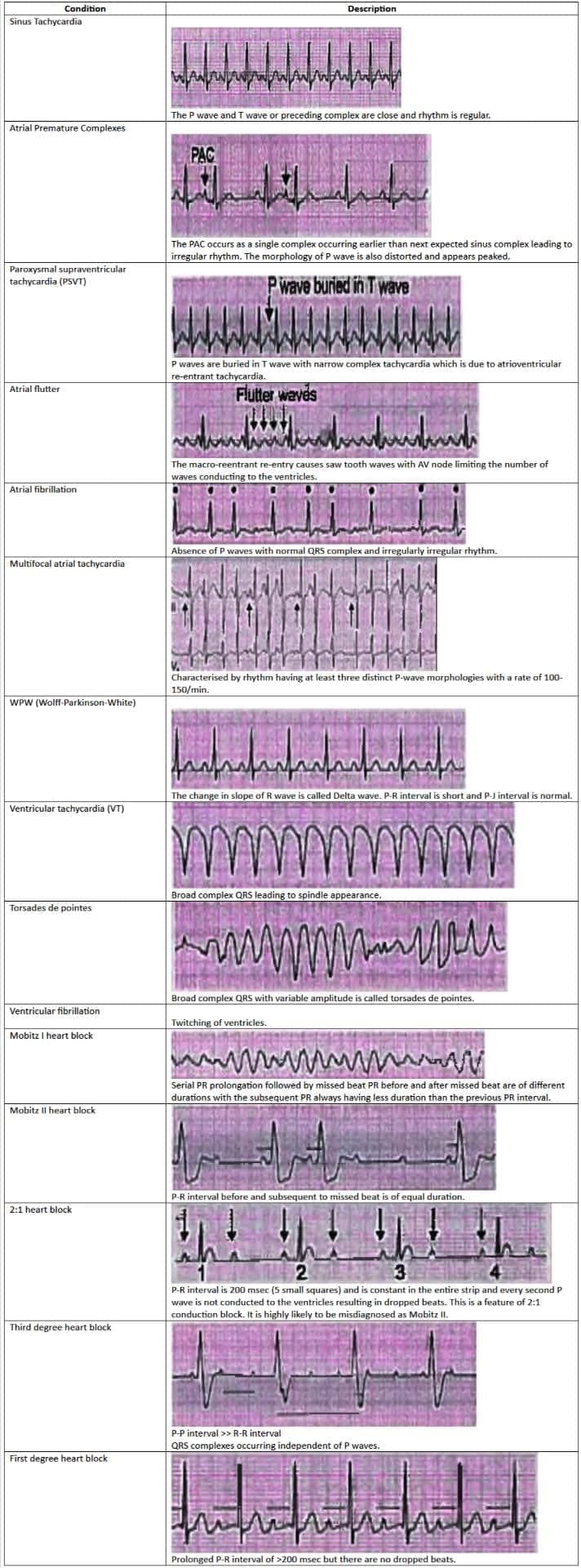
Tachyarrhythmias
- Atrial Fibrillation is the most common sustained arrhythmia.
- Atrial Premature Contraction is the most frequently seen benign rhythm.
- In patients with Chronic Obstructive Pulmonary Disease (COPD), the most common arrhythmia is Multifocal Atrial Tachycardia (MAT).
- Postoperative Atrial Fibrillation is treated with landiolol hydrochloride.
- Atrial Fibrillation can progress to Ventricular Fibrillation if an accessory pathway, such as the bundle of Kent in Wolff-Parkinson-White (WPW) syndrome, conducts antegradely.
- The most common type of Paroxysmal Supraventricular Tachycardia (PSVT) is Atrioventricular Nodal Reentrant Tachycardia (AVNRT), rather than Atrioventricular Reentry Tachycardia (AVRT).
- Ventricular Tachycardia (VT) Storm or electrical storm refers to three or more episodes of VT within 24 hours.
- The most commonly found arrhythmia in cardiac arrest patients is Ventricular Fibrillation.
- The most prevalent genetic cardiovascular disorder is Hypertrophic Obstructive Cardiomyopathy.
- The key causes of sudden death in Hypertrophic Cardiomyopathy (HCM) are Polymorphic Ventricular Tachycardia (VT) and Ventricular Fibrillation (VF).
Mechanism of Arrhythmia
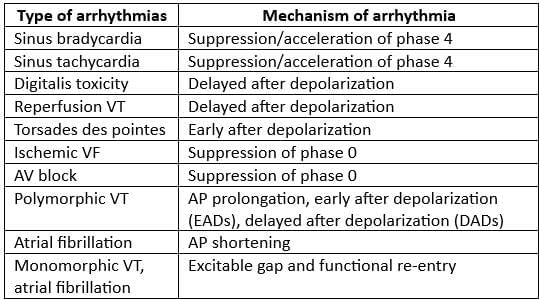
Comparison of Characteristics of Atrial Fibrillation and Atrial Flutter
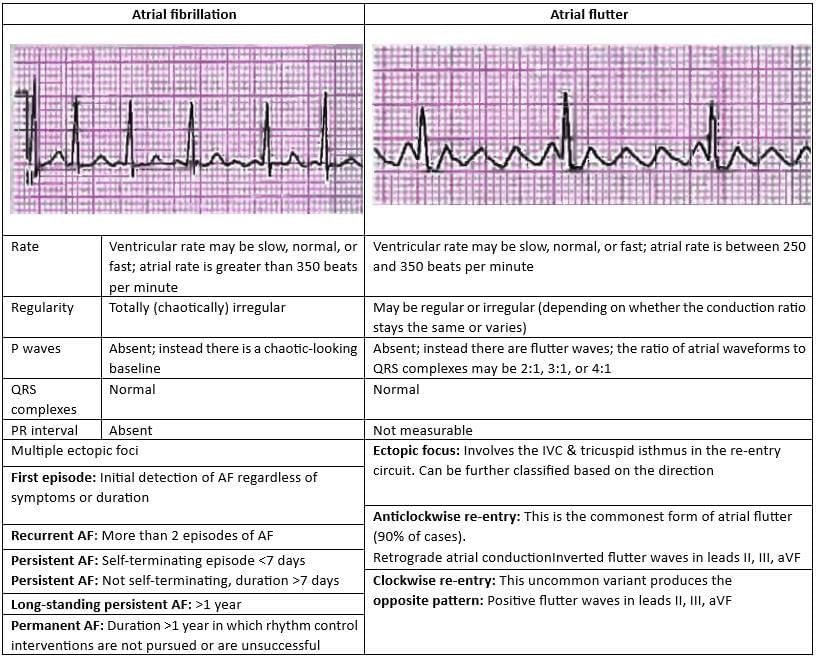 Comparison of Treatment Protocol for Atrial Fibrillation and Atrial Flutter
Comparison of Treatment Protocol for Atrial Fibrillation and Atrial Flutter
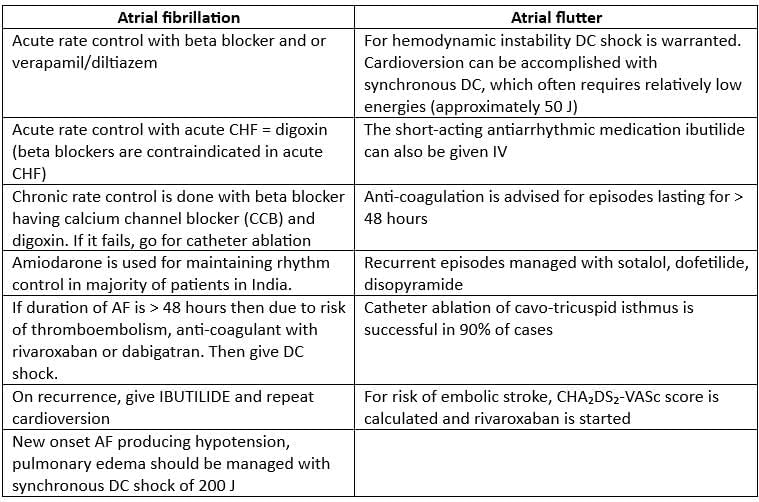
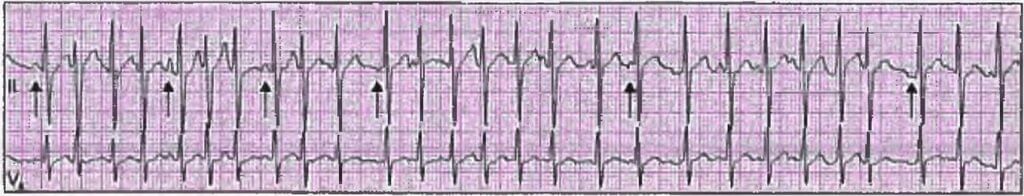
Multi-focal Atrial Tachycardia
- Multi-focal Atrial Tachycardia is characterized by a rhythm that includes at least three different shapes of P-waves, occurring at a rate between 100 to 150 beats per minute.
- There is a noticeable isoelectric pause between the P waves.
- This condition is triggered by automaticity and is commonly observed in patients with Chronic Obstructive Pulmonary Disease (COPD).
Treatment
- DC shock is not advisable for this condition.
- Instead, the focus should be on addressing the underlying issue.
- Calcium channel blockers, such as verapamil or diltiazem, may be effective in reducing the rates of both atrial and ventricular activity.
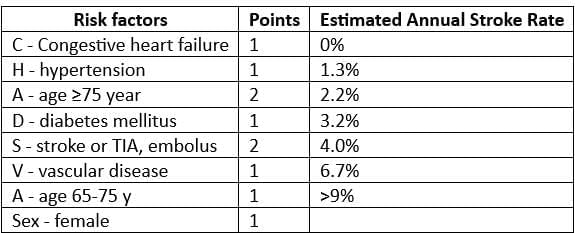
Risk Assessment in Atrial Fibrillation (CHA2 DS2 - VASc) and Need for Anticoagulants
Treatment of Atrial Fibrillation with Wolff-Parkinson-White Syndrome
- In cases where a patient with Atrial Fibrillation and Wolff Parkinson White (WPW) syndrome is hemodynamically unstable, urgent synchronized direct current (DC) cardioversion is required to restore normal heart rhythm.
- It is important to avoid the use of atrioventricular (AV) nodal blocking medications such as adenosine, calcium channel blockers, or beta-blockers in these patients.
- Using these medications can enhance conduction through the accessory pathway, leading to an increased ventricular rate and the risk of progressing to ventricular tachycardia (VT) or ventricular fibrillation (VF), which are life-threatening arrhythmias.
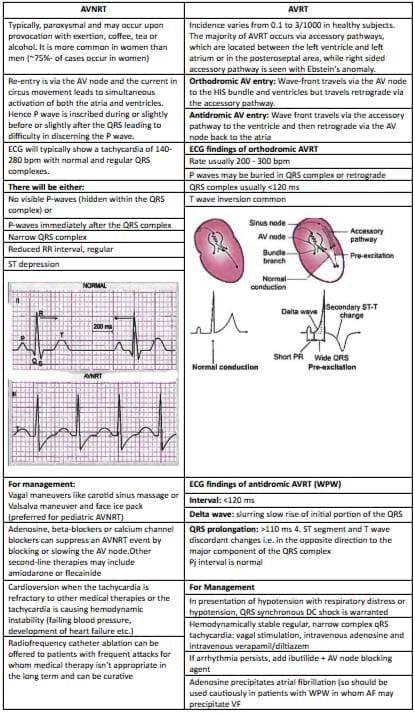
Comparing AVNRT and AVRAVNRT (Atrioventricular Nodal Re-entrant Tachycardia)
Wolff-Parkinson-White Syndrome
- In Wolff-Parkinson-White (WPW) syndrome, the Bundle of Kent causes the ventricles to contract too early, before they are fully filled with blood. This leads to a lower cardiac output and can cause episodes of fainting.
- WPW syndrome can conduct electrical impulses in both directions:
- Antegrade conduction through the Bundle of Kent is known as orthodromic conduction.
- Retrograde conduction to the atria via the His-Purkinje system is referred to as antidromic AV re-entry.
- The delta wave seen in an ECG indicates a change in the slope of the ascending part of the R wave.
- Medications like procainamide and ibutilide can be effective, but their safety and success depend on the patient’s specific condition during rapid pre-excited tachycardia.
- Using AV nodal blocking agents such as verapamil, diltiazem, and adenosine can enhance conduction through the Bundle of Kent, making these drugs unsuitable for patients with Wolff-Parkinson-White syndrome.
- Invasive electrophysiological studies assess whether the pathway can handle dangerously fast heart rates, and catheter ablation can be performed at the same time.
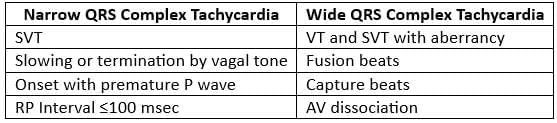
Major Features Differentiating Wide QRS Complex Tachycardia from Narrow Complex Tachycardia
Monomorphic Ventricular Tachycardia
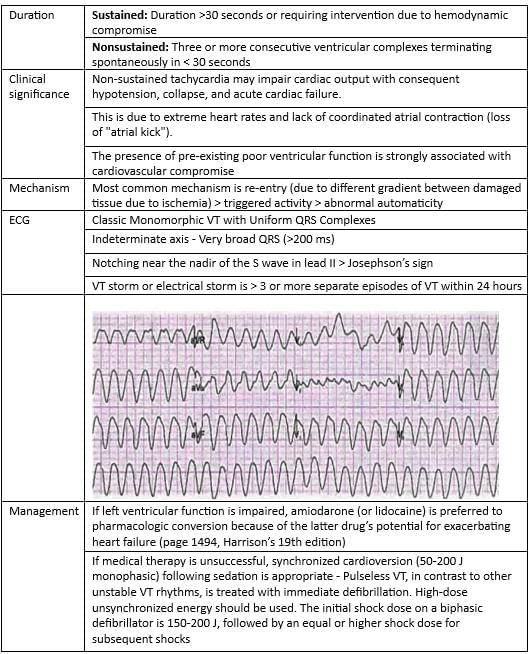
Polymorphic Ventricular Tachycardia
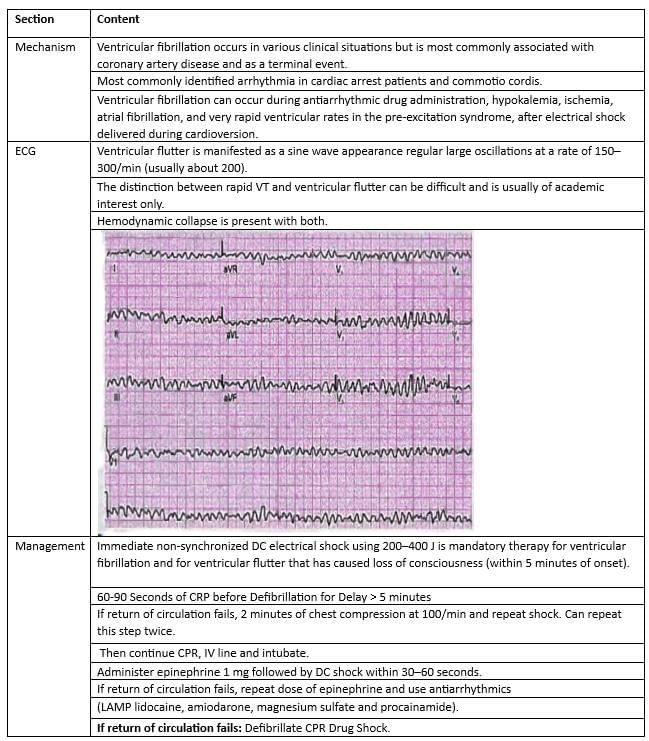
Ventricular Fibrillation
Ventricular Tachycardia
Acute ventricular tachycardia
- Heart is structurally normal and has triggered activity
- Short-acting beta blocker
- Post-myocardial infarction sustained VT
- Lignocaine
- Post-myocardial infarction sustained VT with hypotension
- Synchronized cardioversion
- 100-200 J
- Stable ventricular tachycardia
- Amiodarone. Procainamide
- Chronic recurrent ventricular tachycardia
- Sustained VT with structural heart disease
- Beta blockers are preferred; avoid using anti-arrhythmic drugs in your treatment plan.
- Internal cardioverter defibrillator (ICD)
- Nonsustained VT is defined as more than 3 premature ventricular beats occurring for less than 30 seconds in patients with structural heart disease.
- Lignocaine is not usually used as a preventive measure for VT, although it may have specific uses.
Cardiac Arrest Score
The cardiac arrest score is calculated based on three important factors for patients who have experienced a witnessed out-of-hospital cardiac arrest.
- Emergency department (ED) systolic blood pressure
- Time to return of spontaneous circulation (ROSC) after loss of consciousness
- Neurologic responsiveness
Here’s how the score is determined:
- ED SBP: If the systolic blood pressure in the emergency department is greater than 90 mm Hg, it scores 1 point. If it is 90 mm Hg or lower, it scores 0 points.
- Time to ROSC: If the time to return of spontaneous circulation is 25 minutes or less, it scores 1 point. If it is more than 25 minutes, it scores 0 points.
- Neurologic responsiveness: If the patient is neurologically responsive, it scores 1 point. If the patient is comatose, it scores 0 points.
Bradyarrhythmias
1st Degree Heart Block
- The normal range for the PR interval is between 120 to 200 milliseconds, which is equivalent to 3 to 5 small squares on an ECG.
- In 1st degree heart block, the PR interval is prolonged, and the P wave may be obscured within the preceding T wave.
Causes of 1st Degree Heart Block:
- Increased vagal tone: This refers to heightened activity of the vagus nerve, which can slow down heart rate and affect conduction.
- Athletic training: Well-trained athletes often exhibit increased vagal tone, which can lead to variations in heart conduction.
- Mitral valve surgery: Surgical procedures involving the mitral valve can impact the heart's electrical system.
- Myocarditis: Inflammation of the heart muscle, such as in Lyme disease, can disrupt normal heart rhythms.
- Electrolyte disturbances: Conditions like hyperkalemia, an excess of potassium in the blood, can affect cardiac conduction.
- AV nodal blocking drugs: Medications such as beta blockers, calcium channel blockers, digoxin, and amiodarone can interfere with the conduction through the AV node.
Mobitz I Heart Block / Wenckebach Phenomenon
- In Mobitz I heart block, also known as the Wenckebach phenomenon, there is a gradual lengthening of the PR interval until a P wave is missed.
- The P-R interval is the longest just before the missed beat and the shortest right after it.
- This condition can be triggered by medications such as beta blockers, calcium channel blockers, digoxin, and amiodarone.
- Increased vagal tone, which is common in athletes, can also lead to Mobitz I.
- It may be observed in cases of inferior myocardial infarction or can develop after cardiac surgery, such as mitral valve repair or tetralogy of Fallot repair.
- Mobitz I heart block does not require pacing.
Mobitz II Heart Block
- Mobitz II Heart Block is identified by a normal PR interval followed by a sudden drop in conduction.
- It involves intermittent blocked impulses, usually due to problems in the bundle of His-Purkinje system, which is located below the AV node.
- Mobitz II is often associated with structural damage in the conducting system, such as infarction, fibrosis, or necrosis, unlike Mobitz I, which is linked to functional suppression of AV conduction.
Causes of Mobitz II
- Septal infarction leading to necrosis of the bundle branches.
- Idiopathic fibrosis of the conducting system, known as Lenegre's or Lev's disease.
- Cardiac surgery, particularly procedures near the septum, such as mitral valve repair.
- Inflammatory conditions like rheumatic fever, myocarditis, and Lyme disease.
- Autoimmune diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis.
- Infiltrative myocardial diseases including amyloidosis, hemochromatosis, and sarcoidosis.
- Hyperkalemia, an elevated level of potassium in the blood.
Complete Heart Block (Third Degree Heart Block)
- Atrial Rate: Approximately 100 beats per minute (bpm).
- Ventricular Rate: Approximately 40 bpm.
- Independent Function: The atrial and ventricular rates function independently, indicating that no atrial impulses are reaching the ventricles.
- Inferior Myocardial Infarction: This condition can lead to various complications, including complete heart block.
- AV-Nodal Blocking Drugs: Medications such as calcium-channel blockers, beta-blockers, and digoxin can contribute to heart block.
- Idiopathic Degeneration: Conditions like Lenegre's or Lev's disease, which involve the degeneration of the heart's conducting system, can lead to complete heart block.
- Metabolic and Endocrine Issues: Conditions such as hypothyroidism, adrenal insufficiency, hyperkalemia, and sarcoidosis can be associated with heart block.
- Urgent Pacing: Patients with complete heart block require immediate temporary pacing to manage their condition.
- Permanent Pacing: Following initial management, patients will need permanent pacemaker implantation to regulate their heart rhythm.
Understanding BRUGADA Syndrome
BRUGADA syndrome is an inherited condition passed down in an autosomal dominant manner, primarily caused by a mutation in the SCN5A gene. This genetic issue leads to a decrease in the inward current within the epicardium of the right ventricular (RV) outflow tract.
The disparity in potential between the normal endocardium and the RV outflow tract epicardium triggers a re-entry phenomenon, which can result in life-threatening tachyarrhythmias. BRUGADA syndrome is notably the most common cause of sudden and unexpected death in South Asian men.
Individuals with BRUGADA syndrome may experience symptoms such as a history of palpitations and syncope, and there might be a familial history of sudden death associated with the condition.
ECG Findings and Treatment Options
- A key characteristic observed in ECG readings of individuals with BRUGADA syndrome is the presence of coved ST segment changes accompanied by T wave inversion.
- The Implantable Cardioverter-Defibrillator (ICD) is the preferred treatment option for managing recurrent ventricular arrhythmias and preventing sudden death in affected individuals.
Brugada Sign vs Brugada Syndrome
- Brugada Sign. This is used to diagnose a type of fast heart rhythm known as ventricular tachycardia.
- Brugada Syndrome. This is a genetic condition caused by a defect in the SCN5A gene, which can lead to sudden cardiac death.
- The normal duration of the QRS complex is between 80-100 ms.
- A coved ST segment in leads V1 and V2, along with T wave inversion, is indicative of ventricular tachycardia.
Coronar Blood Supply to the Heart
Coronary Arteries: The heart is supplied with blood by the right and left coronary arteries, which originate from the root of the aorta. Right Coronary Artery: Supplies the following branches:
- Acute marginal branches
- Atrioventricular nodal artery
- Posterior interventricular artery (also known as the posterior descending artery)
Left Main Coronary Artery: This artery branches into:
- Left Anterior Descending (LAD) artery: Supplies the front part of the heart and is often affected by atherosclerosis.
- Septal branches: Supply the interventricular septum.
- Diagonal branches: Supply the anterior wall of the heart.
Left Circumflex Artery: Supplies the lateral and posterior aspects of the heart and branches into obtuse marginal branches.
- Coronary Dominance: Refers to which coronary artery supplies the most blood to the heart. This is usually the right or left coronary artery.
- Atherosclerosis: The left anterior descending artery (LAD) is most commonly affected by atherosclerosis in the heart. The abdominal aorta is the vessel most commonly affected by atherosclerosis overall.
- Internal Mammary Artery: This artery is the least commonly affected by atherosclerosis.
Coronary Dominance
- Right-dominant: In about 80% of people, the posterior interventricular (PIV) artery and at least one posterolateral branch originate from the right coronary artery (RCA).
- Left-dominant: In around 15% of individuals, the PIV and at least one posterolateral branch arise from the left circumflex artery (LCX).
- Balanced: About 5% of cases have a dual supply to the posteroinferior left ventricle (LV) from both the RCA and LCX.
Blood Supply
- The sinoatrial (SA) node receives its blood supply from the SA nodal artery, which comes from the right coronary artery (RCA) in 60% of cases and the left coronary artery (LCA) in 40% of cases.
- Most venous blood from the heart drains into the right atrium (RA) via the coronary sinus. A small amount, however, drains through the thebesian veins into all four heart chambers, creating a natural right-to-left shunt.
Coronary Arteries
- Right Coronary Artery (RCA)
- Left Main Coronary Artery (LCA)
- Left Circumflex Artery (LCX)
- Left Anterior Descending (LAD)
- Septal Perforator Arteries
- Acute Marginal Arteries
- Obtuse Marginal Arteries
- Atrioventricular (AV) Nodal Artery
- Posterior Interventricular Artery
- Diagonal Arteries
Coronary Circulation
Coronary circulation refers to the blood flow to the heart muscle itself, delivered by the coronary arteries. Understanding coronary circulation is crucial for identifying which blood vessel is blocked during an acute myocardial infarction (heart attack).
- Blockages in coronary arteries are indicated by an elevation in the corresponding ECG leads.
Anterior Wall Myocardial Infarction (MI)
- ECG Leads: V1-V6, aVL
- Affected Artery: Left Anterior Descending (LAD) artery, particularly the septal branch
Septal Myocardial Infarction (MI)
- ECG Leads: V1-V6, aVL
- Affected Vessels: Left Circumflex (LCX) artery or diagonal branch
Lateral Wall Myocardial Infarction (MI)
- ECG Leads: V1-V6, aVL
- Affected Vessel: Main left coronary artery
Extensive Anterior Wall Myocardial Infarction (MI)
- ECG Leads: V1-V6, aVL
- Affected Vessels: Left Circumflex (LCX) artery or posterior descending artery
Posterior Wall Myocardial Infarction (MI)
- ECG Leads: V1-V6
- ST Elevation (STE) or ST Depression (STD)
Inferior Wall Myocardial Infarction (MI)
- ECG Leads: Lead II, III, aVF
Ischemic Heart Disease (IHD)
The Framingham risk calculator is used to assess the risk of atherosclerotic cardiovascular heart disease based on various parameters.
- A
- B
- C
- D
- S2
- Age
- Cholesterol
- HDL
- Smoking
Epidemiology
Symptomatic IHD is most prevalent in:
- Men aged 50-60 years
- Women aged 60-70 years
Risk Factors for Atherosclerotic Heart Disease
Major Risk Factors
- Diabetes mellitus
- Family history of myocardial infarction (MI)
- First-degree male relative with MI under 55 years
- First-degree female relative with MI under 60 years
- Hyperlipidemia
- Male or postmenopausal female
- Obesity
- Sedentary lifestyle
- Homocystinemia
The most common lipid profile issue is Familial Combined Hyperlipidemia, affecting 1 in 100-200 individuals.
Markers for Atherosclerosis
- High sensitivity C-reactive protein (CRP). predictor for future coronary events
- Total cholesterol/HDL ratio. >3.5
- Lipoprotein A/apolipoprotein B
- Elevated homocystine levels. can lead to premature atherosclerosis
Chronic Stable Angina/Reversible Ischemia
Chronic stable angina, also known as reversible ischemia, occurs when more than 70% of the diameter inside a coronary artery is narrowed due to atherosclerosis. This condition leads to reduced blood flow to the heart, causing symptoms.
Signs and Symptoms
- Chest Pain: Discomfort or pain in the chest, often behind the breastbone, which may radiate to the left shoulder, arm, neck, or jaw.
- Associated Symptoms: Sweating, nausea, and feelings of anxiety may accompany the chest pain.
- Triggers: Episodes are commonly triggered by the '3 Es': Exertion, Emotion, and Eating.
- Duration: These episodes typically last less than 10-15 minutes and improve with rest.
- Levine's Sign:. characteristic gesture where individuals clutch a fist over their chest when describing the pain.
- Angina Equivalents: Conditions similar to angina, known as angina equivalents, include breathlessness (dyspnoea), acute left ventricular failure, and flash pulmonary oedema.
Clinical Evaluation
- Laboratory Tests: Check hemoglobin levels, fasting glucose, and fasting lipid profile.
- Electrocardiogram (ECG): Perform at rest and during episodes of chest pain, if feasible.
- Chest X-ray (CXR): To exclude conditions like heart failure, valvular disease, pericardial disease, aortic dissection or aneurysm, and signs of pulmonary disease.
- Stress Testing:
- Treadmill Test: Follow the standard Bruce protocol. Look for ischemic ST segment changes, such as flat or downsloping ST depression greater than 0.1 mV below baseline, lasting more than 80 milliseconds.
Contraindications to Exercise Testing
Absolute
- Acute myocardial infarction: within the last two days.
- Uncontrolled cardiac arrhythmias: causing symptoms or impacting blood flow.
- Severe aortic stenosis: with associated symptoms.
- Acute aortic dissection.
- Acute myocarditis or pericarditis.
- Acute pulmonary embolism or lung infarction.
- Unstable angina: not previously stabilized by treatment.
- Uncontrolled symptomatic heart failure.
- Left main coronary artery narrowing.
- Significant aortic stenosis: affecting blood flow.
- High-degree atrioventricular block.
- Abnormal electrolyte levels.
- Tachyarrhythmias or bradyarrhythmias.
- Hypertrophic cardiomyopathy: and other forms of outflow tract obstruction.
- Severe uncontrolled hypertension.
- Mental or physical impairments: preventing adequate exercise.
Dobutamine stress echocardiography
This test is conducted after an acute coronary syndrome (ACS) to assess potential treatment options for patients who cannot exercise and have contraindications to treadmill testing.
- Exercise myocardial perfusion imaging (MPI): using Thallium-201 or technetium-99.
- PET scan (Rubidium-82): considered the best test for evaluating hibernating myocardium.
Role of Cardiac Imaging
- Stress cardiac MRI: the preferred initial non-invasive test for patients with symptoms suggestive of coronary artery disease.
- PET scan: with high accuracy, can differentiate between stunned myocardium and scar tissue.
- Electron beam CT scan: useful for measuring coronary artery calcification.
- Gadolinium-enhanced MRI: the most sensitive test for detecting and assessing the extent of infarction.
Treatment of Chronic Stable Angina
- General Measures:
- Lifestyle modification
- Statins
Guidelines for Statins use
- Old guidelines
- New guidelines
- Initiation of treatment
- Start treatment to achieve Target LDLdefined as:
- Normal population: <>
- Peripheral vascular disease: <>
- Diabetes mellitus: <70>
- Start treatment in patients with:
- Atherosclerotic cardiovascular heart disease
- 21 years with LDL > 190 mg/dL
- Diabetes mellitus, 40-75 years with LDL 70-189 mg/dL
- Diabetes mellitus, 40-75 years with LDL 70-189 mg/dL and 7.5% mortality risk
- Strategy:
- The dose was not specified, nor was the level to which LDL should fall.
- Low intensity statin: up to 30% reduction in LDL using Atorvastatin 10-20 mg
- Moderate intensity statin: 30-50% reduction in LDL
- High intensity statin: 50% reduction in LDL using Atorvastatin 80 mg
- Consider anti-platelet therapy with enteric-coated ASA or clopidogrel when ASA is absolutely contraindicated.
- Beta-blockers(first-line therapy - decrease mortality):
- Increase coronary perfusion and decrease demand (HR, contractility) and BP (afterload).
- Cardioselective agents preferred (e.g. metoprolol, atenolol) to avoid peripheral effects.
- Nitrates(symptomatic control, no clear impact on survival):
- Decrease preload (venous dilatation) and afterload (arteriolar dilatation), and increase coronary perfusion.
- Calcium channel blockers (CCBs)(second line or combination):
- Remember: Verapamil/diltiazem combined with beta-blockers may cause symptomatic sinus bradycardia or AV block.
- ACE inhibitors
- Angina patients tend to have risk factors for cardiovascular disease which warrant use of an ACEI (e.g. hypertension, diabetes, proteinuric renal disease, previous MI with LV dysfunction).
- Angiotensin II receptor blockers (ARBs) when ACEIs are contraindicated.
- Ranolazine: believed to affect the transcellular late sodium current.
- Note: Beta-blockers are typically a first-line treatment for chronic stable angina, but the choice may depend on individual patient factors.
- Major mortality-reducing drugs in chronic stable angina are beta-blockers > statins > aspirin.
Invasive Strategies: Revascularization with percutaneous coronary intervention (PCI) and stenting
- Protocol for Invasive strategies:
- Single vessel disease: PCI with stenting
- Double vessel disease
- Triple vessel disease: CABG
- Stenting is usually done with drug-eluting stents that are coated with products like Paclitaxel, Everolimus, and Sirolimus that prevent redevelopment of atherosclerosis.
Percutaneous Coronary Intervention (PCI)
Percutaneous coronary intervention (PCI) is a medical procedure aimed at relieving significant narrowing in the coronary arteries. The primary methods used in PCI are balloon angioplasty and stenting.
Indications for PCI
- Medically Refractory Angina: Patients with angina that does not respond to medication may have a condition known as hibernating myocardium, where the heart muscle is not receiving enough blood and is functioning at a reduced level.
- Non-ST-Elevation Myocardial Infarction (NSTEMI) and Unstable Angina: PCI is indicated for patients with NSTEMI and unstable angina who have a high risk, as determined by the Thrombolysis in Myocardial Infarction (TIMI) risk score, within 90 minutes of presentation.
- ST-Elevation Myocardial Infarction (STEMI): Primary or rescue PCI is essential for treating STEMI, a severe type of heart attack where there is a complete blockage of a coronary artery.
Coronary Artery Bypass Graft (CABG) Surgery
Significant Left Main Artery Disease
- The survival benefit of CABG is most pronounced in patients with abnormal left ventricular (LV) function, as indicated by a reduced ejection fraction (EF).
Two-Vessel Disease
- CABG is beneficial for patients with significant proximal left anterior descending (LAD) artery disease and impaired LV function.
Patients with One or Two Vessel Disease
- Those without significant LAD disease who have survived sudden cardiac death or sustained ventricular tachycardia (VT).
- Patients lacking significant LAD disease but possessing a large area of viable myocardium and meeting high-risk criteria on noninvasive tests.
Recurrent Stenosis
- CABG is indicated in cases of recurrent stenosis associated with a large area of viable myocardium or high-risk criteria on noninvasive testing.
Conduits for Coronary Artery Bypass Grafting (CABG)
- Occlusion. Patency Rate
- Saphenous vein grafts (SVG)
- At 10 years: 50% occluded, 25% stenotic, 25% angiographically normal
- Left internal thoracic. mammary artery (LITA/LIMA)(LIMA to LAD)
- 90-95% patent at 15 years
Variant Angina (Prinzmetal's Angina)
- Myocardial ischemia caused by coronary artery vasospasm, which can occur with or without atherosclerosis.
- This condition typically arises between midnight and 8 AM, is not triggered by physical activity, and is alleviated by nitrates.
- Variant angina is more prevalent in women, particularly those with a history of migraine or Raynaud's phenomenon.
- Electrocardiogram (ECG) findings usually reveal ST segment elevation, making this type of angina unique in its consistent portrayal of ST segment elevation.
- Many individuals may experience multiple episodes of asymptomatic ST segment elevation, also known as silent ischemia.
- Spasms are frequently observed in the right coronary artery, and spasm is defined as more than 75% blockage in any of the coronary arteries.
- Diagnosis is often established through provocative testing using ergot vasoconstrictors, although this practice is infrequent.
- Treatment options include nitrates and calcium channel blockers (CCBs).
- Upon admission, initial treatment involves nitroglycerin (NTG) drip, followed by the use of long-acting nitrates and CCBs upon discharge.
Acute Coronary Syndrome (ACS)
- Coronary atherosclerosis occurs when a thrombus forms on a ruptured plaque in the coronary arteries.
- Coronary thromboembolism can happen due to conditions like infective endocarditis and paradoxical embolism, but it does not occur due to cholesterol embolism.
- Severe coronary vasospasm is also a contributing factor to ACS.
- Increased demand on the heart, such as in cases of tachycardia or anaemia, can also play a role in ACS.
Spectrum of ACS
- One potential outcome of Acute Coronary Syndrome (ACS) is sudden cardiac death.
- Unstable Angina (UA). Non-ST Elevation Myocardial Infarction (NSTEMI)
Unstable Angina (UA) and Non-ST Elevation Myocardial Infarction (NSTEMI)
- Unstable anginais characterized by any of the following:
- Accelerating pattern of angina: increased frequency, duration, and reduced response to treatment.
- Angina at rest.
- New onset angina.
- Angina following myocardial infarction (MI) or invasive procedures such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
- NSTEMIis diagnosed by the presence of two out of the following three criteria:
- Symptoms indicative of angina or myocardial ischemia.
- Rise and fall of serum markers indicating myocardial necrosis.
- Development of ischemic changes on the ECG without ST segment elevation or new left bundle branch block (LBBB).
ST Elevation Myocardial Infarction (STEMI)
- STEMI occurs when there is an acute rupture of atherosclerotic plaque in the coronary arteries, leading to the formation of a thrombus (blood clot) that completely blocks the coronary artery. This blockage results in myocardial necrosis, which is the death of heart muscle tissue due to lack of blood supply.
- The condition is characterized by diffuse chest pain that lasts for more than 20 minutes. In addition to the chest pain, at least one of the following criteria must be met:
- ECG criteria. There must be ST elevation in two adjacent leads on the electrocardiogram (ECG). This is defined as ST elevation greater than 1 mm in the limb leads or greater than 2 mm in the precordial leads. Alternatively, a new left bundle branch block (LBBB) on the ECG can also indicate STEMI.
- Troponin I levels. Troponin I levels in the blood must be doubled or tripled above the 99th percentile upper reference limit. Troponin is a protein released into the bloodstream when the heart muscle is damaged, and elevated levels indicate myocardial injury.
- Intraoperative myocardial infarction can be diagnosed during surgery using transesophageal echocardiography. This imaging technique may reveal stunned myocardium, which refers to areas of the heart muscle that are temporarily impaired but not permanently damaged.
- Angiography, a medical imaging technique used to visualize the inside of blood vessels, may show the presence of an intracoronary thrombus, further confirming the diagnosis of STEMI.
ECG Alterations in Infarction
- Acute Phase. Occurring in recent days to weeks (typically within 3-5 hours) - Characterized by T wave inversion and ST segment elevation.
- Old Phase. Present for months to years (usually over 6 months) - Marked by significant Q waves.
- Hyperacute Phase. Hyperacute T waves emerge in leads facing the infarcted area or exhibit T wave inversion.
- ST Elevation (Pardee sign) occurs in leads facing the infarcted region, usually within the first hour after the infarct.
- Significant Q Waves develop hours to days after the infarct.
Cardiac Biomarkers
- Troponin T. Levels vary with time, indicating either early reperfusion or permanent occlusion.
- Elevated Enzymes. Commonly seen in conditions like myocardial infarction, congestive heart failure, atrial fibrillation, acute pulmonary embolism, myocarditis, chronic renal insufficiency, sepsis, and hypovolemia.
- CK-MB. Peaks at 1 to 3 days. Elevations can occur due to myocardial infarction, myocarditis, pericarditis, muscular dystrophy, and cardiac defibrillation.
- Heart Fatty Acid Binding Protein. Increases first after a myocardial infarction, followed by myoglobin.
- Reinfarction Indicators. A new rise in CK-MB after 72 hours can suggest reinfarction, with the best identification achieved by a 20% increase over baseline Troponin I values.
- New Biomarkers. Copeptin and heart fatty acid binding protein rise within the first hour in acute coronary syndrome.
- LDH Patterns. Under normal conditions, LDH 1 predominates in serum, while LDH 2 is found in the heart. In cases of myocardial infarction, LDH 1 levels exceed LDH 2 due to myocardial necrosis, resulting in the "flipped pattern."
Universal Definition of Myocardial Infarction
- Type 1: Refers to spontaneous myocardial infarction (MI) due to ischemia resulting from a primary coronary event like plaque rupture, fissuring, or dissection.
- Type 2: Involves MI secondary to ischemia caused by either increased oxygen demand or decreased supply, which may be due to factors such as coronary artery spasm, coronary embolism, anemia, arrhythmias, hypertension, or hypotension.
- Type 3: Pertains to sudden unexpected cardiac death, including cardiac arrest, often with symptoms of myocardial ischemia, even if death occurs before blood samples can be taken or cardiac biomarkers rise.
- Type 4:Involves MI associated with coronary angioplasty or stenting:
- Type 4a: MI linked to percutaneous coronary intervention (PCI), indicated by a fivefold elevation of troponin I.
- Type 4b: MI related to stent thrombosis, documented by angiography or at autopsy.
- Type 5: Involves MI associated with coronary artery bypass grafting (CABG), noted by a tenfold elevation of troponin I.
Acute Management of STEMI
- Thrombolysis (EMS-to-needle) should be done within 30 minutes or Primary PCI (EMS-to-balloon) within 90 minutes.
Management of NSTEMI and STEMI
Role of Dual Antiplatelet Therapy in ACS:
- Guidelines recommend adding a P2Y12 inhibitor to aspirin for all STEMI patients, regardless of the reperfusion method.
- This treatment should continue for 14 days, then up to 1 year.
General measures:
- ABCs: assess and correct hemodynamic status first.
- Bed rest, cardiac monitoring, and oxygen administration.
- Nitroglycerin SL.
- Morphine IV.
Anti-platelet and anticoagulation therapy:
- Acetylsalicylic acid (ASA) 162-325 mg chewed, along with clopidogrel 300 mg loading dose, then 75 mg OD in addition to ASA.
- Subcutaneous low molecular weight heparin (LMWH) or IV unfractionated heparin (UFH) (LMWH is preferred, except in renal failure or if CABG is planned within 24 hours).
- If PCI is planned: Clopidogrel 300 mg loading dose and IV GP IIb/IIIa inhibitor.
- Continue LMWH or UFH, followed by oral anticoagulation at discharge if at high risk for thromboembolic events (e.g., large anterior MI, atrial fibrillation, severe LV dysfunction, CHF, previous DVT or PE, or echo evidence of mural thrombus).
Beta-blockers:
- First dose IV followed by oral administration.
- CCB may be used if there is no severe LV dysfunction and beta-blockers are contraindicated (CCBs do not prevent MI or decrease mortality).
Invasive strategies and re-perfusion options:
- Abciximab, B-blocker, Enoxaparin, Morphine, Oxygen, Aspirin, and Nitrates.
- (Mnemonic: ABE-MOAN )
- If the patient does not improve, proceed with delayed PCI.
STEMI: The preferred treatment is PCI.
- Primary PCI (≤ 12 hours after symptom onset and ≤ 90 minutes after presentation) improves outcomes.
- If the patient arrives at a non-PCI capable hospital, transfer them for primary PCI if the time from the first medical contact to device is ≤ 120 minutes.
- Thrombolysis: Recommended if the patient presents within ≤ 12 hours of symptom onset and ≤ 30 minutes after presentation.
- Thrombolysis is not suitable for unstable angina or NSTEMI unless specific criteria are met.
- The most common complication with thrombolysis is bleeding.
- First medical contact to device time should be < 90="" minutes=""> if PCI is to be performed.
Situations Where Thrombolysis is Not Recommended in STEMI
- Previous bleeding in the brain
- Known issues with blood vessels in the brain
- Known cancer in the brain
- Significant injury to the head or face
- Stroke that happened more than 3 months ago
- Ongoing bleeding
- Suspected tear in the aorta
- Chronic and severe high blood pressure that is hard to control
- Uncontrolled high blood pressure (systolic blood pressure > 180 mm Hg or diastolic blood pressure > 110 mm Hg)
- Current use of blood thinners
- Noncompressible vascular punctures
- Recent internal bleeding (within the last 2-4 weeks )
- Prolonged cardiopulmonary resuscitation or major surgery
- Pregnancy
- Active peptic ulcer
Complications of Myocardial Infarction
- Etiology
- Presentation
- Therapy
- Tachycardia
- Sudden death
- 6-12 hours
- Bradycardia
- Mobitz 2 heart block
- First 48 hours
- Temporary pacing
- Myocardial Rupture
- LV free wall
- Papillary muscle
- Transmural
- 1-7 days
- Septal
- Shock / CHF
- Infarction or within 48 hours
- Inotropes, intra-aortic balloon pump
- Post-infarct angina
- Coronary stenosis and multi-vessel disease
- Anytime
- Aggressive medical therapy, PCI or
- Recurrent MI
- Thrombo-reocclusion
- Mural/apical thrombus (OVT)
- 7-10 days up to 6 months
Heart Failure
Congestive Heart Failure (CHF)
Systolic Dysfunction (Impaired Ventricular Ejection)
- In systolic dysfunction, the heart's ability to pump blood is compromised, leading to a reduced left ventricular ejection fraction (LVEF) and lower stroke volume.
- Common clinical findings include:
- Displaced apex beat: The point of maximum impulse (PMI) is shifted due to heart enlargement.
- Presence of S3 heart sound: An additional heart sound indicative of heart failure.
- Enlarged heart on chest X-ray (CXR): Radiographic evidence of cardiomegaly.
- Approximately 50% of CHF patientsexperience sleep disturbances, including:
- Cheyne-Stokes breathing:. pattern of periodic breathing with apneas.
- Sleep apnea: Can be central (neurological) or obstructive (airway blockage).
Alcohol
- Dilated cardiomyopathy:. condition where the heart's ability to pump blood is decreased due to dilation of the heart chambers, often associated with chronic alcohol abuse.
Diastolic Dysfunction (Impaired Ventricular Filling)
- Diastolic dysfunction refers to heart failure patients with normal systolic function, meaning their ejection fraction is within the normal range.
- In this condition, there is an increase in left ventricular (LV) filling pressures, leading to congestion in both pulmonary and systemic veins.
- Common clinical findings in diastolic dysfunction include:
- Hypertension (HTN): High blood pressure is often present.
- Sustained apex beat: The apex of the heart is palpable and sustained due to increased pressure.
- Presence of S4 heart sound: An extra heart sound indicating impaired ventricular filling.
- Normal-sized heart on CXR: Chest X-ray shows no enlargement of the heart.
- Left ventricular hypertrophy (LVH): Thickening of the heart muscle, visible on ECG or echocardiogram.
- Normal LVEF: Ejection fraction is within the normal range, indicating preserved systolic function.
Causes of Decreased Compliance
- Severe hypertrophy:Thickening of the heart muscle due to conditions such as:
- Hypertension (HTN): High blood pressure leading to increased workload on the heart.
- Aortic stenosis (AS): Narrowing of the aortic valve, obstructing blood flow from the heart.
- Hypertrophic cardiomyopathy (HCM): Genetic condition causing abnormal thickening of the heart muscle.
- Restrictive cardiomyopathy (RCM):. condition where the heart muscle becomes rigid and less compliant, restricting filling during diastole.
High-Output Heart Failure
- High-output heart failure occurs when there is an increased demand for cardiac output, leading to heart failure symptoms.
- While it can exacerbate existing heart failure or be a primary cause in certain instances, it is rarely a primary cause of heart failure.
- Conditions to consider in the differential diagnosis include:
- Anemia: Low red blood cell count leading to decreased oxygen carrying capacity.
- Thiamine deficiency (beriberi): Nutritional deficiency affecting cardiovascular function.
- Hyperthyroidism: Overactivity of the thyroid gland increasing metabolic demands.
- A-V fistula or left-to-right shunting: Abnormal blood vessel connections altering blood flow dynamics.
- Paget's disease: Disorder of bone metabolism affecting blood flow.
- Renal disease: Kidney dysfunction impacting fluid and electrolyte balance.
- Hepatic disease: Liver dysfunction affecting metabolic processes.
Framingham Criteria for Heart Failure
- Acute pulmonary edema
- Ankle edema
- Cardiomegaly
- Hepatomegaly
- Hepatojugular reflux
- Dyspnea on exertion
- Neck vein distention
- Nocturnal cough
- Paroxysmal nocturnal dyspnea or orthopnea
- Pleural effusion
- Systolic/Ventricular group
- Weight loss > 4.5 kg in 5 days
- In response to treatment
- Classification stage of heart failure
- No clinical signs of heart failure
II:
- Rales/crackles in lung, elevated JVP
III:
- Frank acute pulmonary edema
IV:
- Cardiogenic shock or hypotension
Heart failure is diagnosed when 2 major criteria or one major and two minor criteria are met.
Investigations
- N-Terminal Pro B-type Natriuretic Peptide (NT-proBNP): This hormone is typically elevated in cases of heart failure. It plays a crucial role in regulating blood pressure and fluid balance.
- Uric Acid: Levels of uric acid are measured as part of the investigation.
- Blood Urea Nitrogen (BUN): BUN levels are assessed.
- Electrocardiogram (ECG): An ECG is performed to check for various heart conditions, including chamber enlargement, irregular heart rhythms (arrhythmias), and signs of reduced blood flow to the heart (ischemia) or heart muscle damage (infarction).
- Chest X-Ray (CXR):. chest X-ray is conducted to look for signs of heart enlargement (cardiomegaly), fluid accumulation in the pleural space (pleural effusion), redistribution of blood flow in the lungs, Kerley B-lines (indicative of pulmonary congestion), and bronchiolar alveolar cuffing.
- Echocardiography: This test assesses the left ventricular ejection fraction (LVEF), cardiac dimensions, blood flow or wall motion abnormalities, valvular heart disease, and the presence of fluid around the heart (pericardial effusion).
- Radionuclide Angiography (MUGA): This imaging technique is used to evaluate LVEF.
- Myocardial Perfusion Scintigraphy: This test involves the use of thallium or sestamibi SPECT (Single Photon Emission Computed Tomography) to assess blood flow to the heart muscle during rest and stress conditions.
Diagnostic Evaluation
- Cardiac output and perfusion of extremities
- (elevated PCWP )
What to be done?
- Warm to touch (wet lungs)
- Decrease pulmonary edema
- Use diuretics
- Cold to touch
- Increase the cardiac output
- Administer dobutamine. dopamine
- Use inodilators to unload (dry lungs)
- Pulmonary or hepatic disease
- Treat lung disease
- RV dysfunction
- Fluid bolus
Management of Heart Failure with Preserved Ejection Fraction
- Beneficial Drugs:
- Sacubitril and Valsartan: Recently approved combination showing early promise.
- Aldosterone Antagonists: Improve clinical outcomes in patients.
- ARNIs (Angiotensin Receptor Neprilysin Inhibitors): More effective than ACE inhibitors like Enalapril in reducing cardiovascular death and hospitalizations for heart failure by 20% in patients with heart failure and reduced LVEF.
- ACE Inhibitors: Play a role in both preserved and reduced LVEF, contributing to prevention.
- Beta-Blockers: Metoprolol, Bisoprolol, Carvedilol are commonly used.
Acute Pulmonary Edema
- Address acute triggers like ischemia and arrhythmias.
- Long-Acting Muscarinic Antagonist. LAMA. , flogges .
- Adjust Lasix. 40-500 mg IV ) based on individual patient needs.
- Administer Morphine. 2-4 mg IV ) to alleviate anxiety and reduce preload.
- Clarify the use and effects of Nitroglycerine. topical/IV/SL ) .
- Provide Oxygen therapy.
- Utilize Positive Airway Pressure. CPAP/BIPAP ) to decrease preload and minimize ventilation requirements.
- Position the patient sitting up with legs hanging down, unless they are hypotensive.
Cardiogenic Shock
- Administer Norepinephrine, Dobutamine, or Dopamine infusion.
- Intra-aortic balloon pump. IABP ) is rarely used but can be life-saving.
- Consider Left or right ventricular assist device. LVAD/RVAD ) in critical cases.
- Cardiac transplant may be necessary in severe cases.
Procedural Interventions for Congestive Heart Failure (CHF)
- Resynchronization Therapy. This therapy involves the use of a biventricular pacemaker to alleviate symptoms.
- Indications for Resynchronization Therapy:
- QRS duration exceeding 120 msec
- Left ventricular ejection fraction (LVEF) below 35%
- Severe symptoms persisting despite optimal treatment
- Implantable Cardioverter Defibrillator (ICD). An ICD can improve survival by preventing sudden cardiac death, both as a primary and secondary intervention.
- Valve Repair. This option is suitable for patients who can undergo surgery and have significant valve disease contributing to congestive heart failure (CHF).
- Algorithm for Treatment of CHF.
- Confirm the diagnosis of heart failure (HF)
- Assess for fluid retention
- Use diuretic therapy as necessary
- Implement ICD for patients with NYHA Class II-III
- Consider cardiac resynchronization therapy (CRT) for NYHA Class III-IV patients with QRS > 120 ms
- Use angiotensin receptor blockers (ARB) if patients are intolerant to ACE inhibitors
- For patients without fluid retention, initiate ACE inhibitor therapy
- Consider adding beta blockers, ARB, aldosterone antagonists, hydralazine/isosorbide, or digoxin as needed
- For patients in NYHA Class I-IV, address persistent symptoms or specific needs
- ACE Inhibitors. Patients on ACE inhibitors may experience an increase in serum creatinine or potassium levels but do not need to discontinue the medication. ACE inhibitors can be continued even if serum creatinine reaches 3 mg/dL.
- Spironolactone. Discontinue spironolactone if serum creatinine levels become excessively high.
Myocardial Disease
Myocardial disease involves the inflammation of the heart muscle, known as the myocardium. This condition can range from being acute (short-term) to chronic (long-lasting) and is a significant contributor to dilated cardiomyopathy, a condition where the heart becomes enlarged and weakened.
The heart muscle, or myocardium, can become inflamed due to various factors, including infections, toxins, allergic reactions, and systemic diseases. This inflammation can impair the heart's ability to pump blood effectively, leading to heart failure and other complications.
Causes of Myocardial Disease
1. Viral Infections
- Coxsackie B Virus:. common viral cause of myocarditis.
- Echovirus: Another virus linked to heart muscle inflammation.
- Poliovirus: Known for causing poliomyelitis, it can also affect the heart.
- HIV: The virus responsible for AIDS can lead to myocarditis.
- Mumps Virus: Can cause myocarditis in some cases.
2. Bacterial Infections
- Staphylococcus aureus:. bacterial infection that can affect the heart.
- Clostridium perfringens: Associated with gas gangrene, it can also infect the heart.
- Corynebacterium diphtheriae: The bacterium causing diphtheria, which can lead to myocarditis.
- Mycoplasma pneumoniae: Known for causing pneumonia, it can also affect the heart.
3. Other Infectious Agents
- Fungi: Fungal infections can lead to myocarditis.
- Spirochaetes: Such as Borrelia burgdorferi, the causative agent of Lyme disease.
- Chagas Disease: Caused by Trypanosoma cruzi, leading to heart muscle inflammation.
- Toxoplasmosis: An infection caused by Toxoplasma gondii that can affect the heart.
4. Toxic Reactions
- Catecholamines: Excessive levels can be toxic to the heart.
- Chemotherapy: Certain drugs used in cancer treatment can damage the heart.
- Cocaine:. recreational drug that can have toxic effects on the heart.
5. Hypersensitivity Reactions
- Drugs: Antibiotics, diuretics, lithium, and clozapine can trigger allergic reactions affecting the heart.
- Insect or Snake Bites: Can cause allergic reactions leading to myocarditis.
6. Systemic Diseases
- Collagen Vascular Diseases: Such as Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA).
- Sarcoidosis: An inflammatory disease that can affect the heart.
- Autoimmune Conditions: Various conditions where the immune system attacks the body’s tissues, including the heart.
7. Other Conditions
- Giant Cell Myocarditis:. rare and severe form of myocarditis.
- Acute Rheumatic Fever:. complication of untreated strep throat that can affect the heart.
- Constitutional Illnesses: Various systemic illnesses that can affect the heart.
- Acute Congestive Heart Failure (CHF):. sudden worsening of heart failure symptoms.
Symptoms and Complications
1. Chest Pain: Can result from
- Pericarditis: Inflammation of the pericardium, the outer lining of the heart.
- Cardiac Ischemia: Reduced blood flow to the heart muscle.
2. Complications
- Arrhythmias: Abnormal heart rhythms.
- Systemic or Pulmonary Emboli: Blood clots that can travel to the lungs or other parts of the body.
- Sudden Death: Due to severe heart dysfunction.
Diagnosis
1. Electrocardiogram (ECG) Findings
- Non-Specific ST-T Changes: Abnormalities in the ST segment or T wave of the ECG.
- Conduction Defects: Abnormalities in the electrical conduction system of the heart.
2. Laboratory Tests
- Increased Levels of:CK: Creatine Kinase, an enzyme indicating muscle damage. Troponin:. protein indicating heart muscle injury. LDH: Lactate Dehydrogenase, an enzyme that can indicate tissue damage. AST: Aspartate Aminotransferase, an enzyme that can indicate liver or heart damage.
- Other Possible Increases:WBC: White Blood Cell count, indicating infection or inflammation. ESR: Erythrocyte Sedimentation Rate, indicating inflammation. ANA: Antinuclear Antibodies, indicating autoimmune disease. Rheumatoid Factor: Indicating rheumatoid arthritis. Complement Levels: Indicating autoimmune disease.
3. Further Tests
- Blood Culture: To identify infections.
- Viral Titres: To identify viral infections.
- Cold Agglutinin Test for Mycoplasma: To identify Mycoplasma infections.
4. Chest X-Ray (CXR) Findings
- Enlarged Cardiac Silhouette: Indicating cardiomegaly or heart enlargement.
5. Echocardiography Findings
- Dilated and Hypokinetic Chambers: Enlarged heart chambers with reduced movement.
- Segmental Wall Motion Abnormalities: Abnormal movement in specific areas of the heart wall.
6. Myocardial Biopsy
- Reserved for limited cases to obtain tissue samples for diagnosis.
Management
- Supportive Care: Providing necessary support to the patient.
- Restricting Physical Activity: Limiting physical exertion to reduce the heart's workload.
- Treating Congestive Heart Failure (CHF): Managing symptoms and complications of heart failure.
- Treating Arrhythmias: Managing abnormal heart rhythms.
- Anticoagulation: Preventing blood clots with anticoagulant medications.
- Treating Underlying Causes: Addressing the root cause of myocarditis if possible.
Cardiomyopathy (Takotsubo Cardiomyopathy, Arrhythmogenic RV Dysplasia)
Takotsubo Cardiomyopathy / Broken Heart Syndrome
- The main cause is stress that leads to a large release of catecholamines, which can damage the heart muscle.
- This stress can result in a temporary weakening of the heart's pumping ability.
- A biopsy of the heart muscle reveals areas of cell damage, along with certain inflammatory cells and necrosis.
Modified Mayo Criteria for Takotsubo Cardiomyopathy (T.C.M) / Broken Heart Syndrome
- Temporary reduction or absence of movement in the left ventricle.
- No blockage in the coronary arteries caused by a blood clot.
- Changes such as ST segment elevation, T wave inversion, or slight increases in cardiac troponin levels.
- Exclusion of conditions like pheochromocytoma and myocarditis is crucial for diagnosis.
- All four criteria must be met for a confirmed diagnosis.
Clinical Features
- Symptoms can be triggered by emotionally or physically stressful events, such as being trapped in a lift during a large earthquake.
- Common symptoms include chest pain, dyspnea (shortness of breath), palpitations, nausea, vomiting, syncope (fainting), and cardiogenic shock.
- Hypotension (low blood pressure) occurs due to a decrease in stroke volume and is often accompanied by heart murmurs and crackling sounds (rales) heard during a physical examination.
- Electrocardiogram (ECG) findings may show ST segment elevation along with elevated cardiac troponin levels, which can resemble ST-Elevation Myocardial Infarction (STEMI).
- However, during Percutaneous Coronary Intervention (PCI) and angiography, the coronary arteries appear normal.
- A bedside echocardiogram may reveal hypokinesia (reduced movement) of the left ventricle (LV) with ballooning, similar to a jar used to trap an octopus.
- Management includes the use of an intra-aortic balloon pump to address cardiogenic shock, and this condition is unique among cardiomyopathies because recovery typically occurs within a few weeks.
Arrhythmogenic Right Ventricular Dysplasia
- Arrhythmogenic Right Ventricular Dysplasia (ARVD) is a condition characterized by structural abnormalities in the heart, specifically due to fatty infiltration and fibrosis of the right ventricle (RV) myocardium. This leads to progressive dilation and dysfunction of the right ventricle.
- While the left ventricle may also be affected, the interventricular septum remains intact.
- Electron microscopy studies reveal defects in desmosomes, which are essential for cell-to-cell adhesion in the heart muscle.
- The condition is most commonly inherited in an autosomal dominant manner.
- Symptoms often include palpitations and syncope (fainting), and there may be a family history of sudden cardiac death.
- Typical ECG abnormalities seen in ARVD include ventricular ectopic beats, ventricular tachycardia (VT), and ventricular fibrillation.
- Right ventricular dysfunction can lead to symptoms such as dyspnea (shortness of breath) and peripheral edema (swelling) in the legs.
- A key differential diagnosis is Uhl's anomaly, which is characterized by a thin-walled right ventricle due to a lack of RV myocardium. In contrast, ARVD involves the replacement of RV muscle with fibrofatty tissue.
- ECG findings in ARVD may also include the presence of an epsilon wave.
- Cardiac MRI is the preferred imaging modality for diagnosing ARVD.
- Electrophysiological studies may be performed to induce ventricular tachycardia for diagnostic purposes.
- Histopathological findings of fibrosis in ARVD are not specific, as fatty infiltration can also occur in elderly individuals without the disease.
- To prevent sudden cardiac death in patients with sustained ventricular tachycardia or ventricular fibrillation, the implantation of an implantable cardioverter defibrillator (ICD) is necessary.
- Beta blockers are typically the first-line medication for managing ARVD, with sotalol being effective for both inducible and non-inducible ventricular tachycardia.
Hypertrophic Cardiomyopathy (HCM)
HCM is a condition characterized by asymmetric septal hypertrophy and left ventricular outflow tract obstruction. It is a type of diastolic dysfunction that eventually leads to systolic dysfunction. The prevalence of HCM is estimated to be between 1 in 500 and 1 in 1,000 in the general population. This condition is autosomal dominant and is caused by a defect in the beta myosin gene located on chromosome 14.
As a result of elevated filling pressures, pulmonary edema occurs, with dyspnoea often being the first symptom. In many instances, HCM may go unrecognized initially, and patients might only report effort intolerance.
Symptoms
- Angina
- Pre-syncope or syncope due to left ventricular outflow obstruction or arrhythmia
- Congestive heart failure (CHF)
- Arrhythmias
- Sudden cardiac death
Sudden cardiac death can occur during physical activities, such as playing football, as a result of ischemic ventricular fibrillation.
Physical Examination Findings
- Pulses. Rapid upstroke, bifid carotid pulse (Pulsus Bisferiens)
- Precordial palpation. Well-sustained double apical impulse, 'triple ripple' (triple apical impulse in HCM), LV lift
- Precordial auscultation. Normal or paradoxically split S2, S4, harsh systolic diamond-shaped murmur at LLSB or apex, enhanced by squat to standing or Valsalva (murmur due to LVOT obstruction compared to aortic stenosis); often with pan-systolic murmur due to mitral regurgitation
Diagnostic Tests
- ECG. Left ventricular hypertrophy (LVH), high voltages across the precordium, prominent Q waves or tall R waves in V1, and P wave abnormalities
- Echocardiogram. Asymmetric septal hypertrophy (less commonly apical), systolic anterior motion of the mitral valve, and mitral regurgitation
It is important to avoid factors that can increase obstruction, such as volume depletion and strenuous exertion.
Treatment of Hypertrophic Obstructive Cardiomyopathy (HOCM)
Medical Agents:
- Propranolol is the preferred medication as it helps decrease heart rate and reduce oxygen consumption. This, in turn, increases the duration of diastole, allowing for better heart filling and improved cardiac output.
- Verapamil may also be considered as a treatment option.
- Medications such as NTG, digoxin, ACE inhibitors, and diuretics are contraindicated in patients with HOCM.
Management of Patients with Drug-Refractory Symptoms
- Surgical Myomectomy
- Septal Ethanol Ablation
- Dual-Chamber Pacing
Treatment of Ventricular Arrhythmias
- Amiodarone or Implantable Cardioverter-Defibrillator (ICD)
Restrictive Cardiomyopathy (RCM)
Restrictive Cardiomyopathy is a condition where the heart's ventricles lose their flexibility, making it hard for them to fill with blood properly. This stiffness can be due to factors like fibrosis or reduced compliance, but unlike other types of cardiomyopathy, the ventricles in RCM do not enlarge or thicken. Typically, the heart's ability to contract remains normal in this condition. Restrictive Cardiomyopathy is the rarest form of cardiomyopathy.
Causes of Restrictive Cardiomyopathy
- Infiltrative Conditions:These conditions involve the infiltration of abnormal substances into the heart tissue, leading to stiffness. Examples include:
- Amyloidosis: The most common cause of RCM, where amyloid proteins build up in the heart tissue.
- Sarcoidosis: An inflammatory disease that can affect multiple organs, including the heart.
- Non-infiltrative Conditions:These conditions cause stiffness without infiltration of abnormal substances. Examples include:
- Scleroderma:. connective tissue disorder that can lead to fibrosis of the heart.
- Idiopathic Myocardial Fibrosis: Fibrosis of the heart muscle with no known cause.
- Storage Diseases:These diseases involve the abnormal accumulation of substances in the body, which can affect the heart. Examples include:
- Haemochromatosis: Excess iron accumulation in the body.
- Fabry's Disease:. genetic disorder affecting fat metabolism.
- Glycogen Storage Diseases: Disorders affecting glycogen metabolism.
- Endomyocardial Fibrosis:. condition characterized by fibrosis of the endocardium, leading to stiffness.
- Loeffler's Endocarditis:. rare condition involving eosinophilic infiltration of the heart tissue.
- Eosinophilic Endomyocardial Disease: Similar to Loeffler's endocarditis but with eosinophilic infiltration of the endomyocardium.
- Radiation Therapy: Previous radiation therapy can lead to heart complications, including mixed restrictive and constrictive pericarditis.
- Carcinoid Syndrome:. condition that can affect the function of the tricuspid or pulmonary valve, contributing to RCM.
Clinical Manifestations
- Congestive Heart Failure (CHF) is common, often with preserved left ventricular systolic function. Patients may also experience arrhythmias.
- Jugular Venous Pressure (JVP) is raised, showing distinct x and y descents. Kussmaul's sign, which indicates impaired filling of the heart, may also be present.
- Patients might have abdominal distention due to ascites and significant bilateral peripheral edema. They may report discomfort or tenderness in the liver area.
- Chest pain can occur, potentially related to angina or mimicking myocardial ischemia, particularly in patients with amyloidosis. This may be due to compression of small vessels in the heart. Palpitations are common, often caused by atrial fibrillation, which is frequently seen in idiopathic Restrictive Cardiomyopathy (RCM).
- Approximately one-third of patients with idiopathic RCM may experience thromboembolic complications, especially pulmonary emboli. In cases of atrial fibrillation, there is a heightened risk of left atrial clots and systemic emboli.
- Heart sounds S1 and S2 are typically normal, with a characteristic split S2. A loud early diastolic filling sound (S3) may be present but is rare in amyloidosis. A fourth heart sound (S4) is usually absent, possibly due to amyloid infiltration of the atria. Murmurs from mitral and tricuspid valve regurgitation may be audible but are generally not hemodynamically significant.
Constrictive
- History of pericarditis or conditions leading to pericardial disease.
- History of systemic diseases such as amyloidosis or hemochromatosis.
- Peripheral signs indicating systemic disease.
- Systemic examination findings including heart sounds with a pericardial knock and a high-frequency sound.
- Presence of a loud diastolic filling sound (S3), low-frequency sound, and absence of murmurs, though murmurs of mitral and tricuspid insufficiency may be present.
- Normal results from prior chest radiographs.
- ECG Findings. Low voltage, non-specific, diffuse ST-T wave changes, with or without non-ischemic Q waves.
- Chest X-ray (CXR). Mild cardiac enlargement.
- Echocardiogram (Echo) Findings. Left Ventricular Hypertrophy (LVH), Right Ventricular Hypertrophy (RVH), Left Atrial Enlargement (LAE), Right Atrial Enlargement (RAE), and valve thickening.
- Cardiac Catheterization. Increased end-diastolic ventricular pressures.
- Endomyocardial Biopsy. To determine the cause, especially for infiltrative RCM, and is the investigation of choice.
- Differential Diagnosis. Exclude constrictive pericarditis.
- Management. Treat underlying disease, control heart rate, consider anticoagulation if atrial fibrillation is present, provide supportive care and treatment for CHF and arrhythmias.
- Heart Transplant. May be considered for CHF that does not respond to medical therapy.
Dilated Cardiomyopathy (DCM)
- DCM is characterized by the unexplained dilation and reduced pumping capability of one or both ventricles.
- Etiology: Approximately 50% of DCM cases are idiopathic, believed to be caused by viral infections or genetic factors.
- Alcohol consumption is a significant contributing factor to the development of DCM.
- Uncontrolled tachycardia, such as persistent atrial fibrillation, can lead to DCM.
- Collagen vascular diseases like systemic lupus erythematosus (SLE), polyarteritis nodosa (PAN), dermatomyositis, and progressive systemic sclerosis are associated with DCM.
- Infectious diseases including viral infections (e.g., Coxsackie B virus, HIV), Chagas disease, Lyme disease, Rickettsial diseases, and acute rheumatic fever can contribute to DCM.
- Neuromuscular diseases such as Duchenne muscular dystrophy, myotonic dystrophy, and Friedreich's ataxia are linked to DCM.
- Metabolic issues like uremia and nutritional deficiencies (e.g., thiamine, selenium, carnitine) are risk factors for DCM.
- Endocrine disorders including hyperthyroidism, hypothyroidism, diabetes mellitus, and pheochromocytoma can lead to DCM.
- Peripartum cardiomyopathy is a condition associated with DCM.
- Exposure to toxic substances such as cocaine, heroin, and organic solvents can contribute to DCM.
- Drug-related causes include chemotherapies like doxorubicin and cyclophosphamide, as well as anti-retrovirals, chloroquine, and clozapine.
- Sudden cardiac death due to fatal arrhythmias is a leading cause of mortality in DCM.
- Tests: Blood tests including CBC, electrolytes, creatinine, bicarbonate, BNP, CK, troponin, and liver function tests.
- ECG: May reveal ST-T wave changes, poor R wave progression, conduction defects, and arrhythmias like non-sustained ventricular tachycardia (VT).
- CXR: Shows global cardiomegaly and signs of congestive heart failure.
- Echocardiography: Demonstrates 4-chamber enlargement, reduced movement with lowered left ventricular ejection fraction (LVEF), mitral regurgitation (MR), tricuspid regurgitation (TR), and mural thrombus.
- Endomyocardial biopsy: Not routinely performed but used to rule out treatable causes.
- Angiography: Conducted in selected patients to exclude ischemic heart disease.
- Diuretics: Require careful titration as they can lower cardiac output.
- Thromboembolism prophylaxis: Anticoagulation with warfarin is recommended.
- Arrhythmias: Treat symptomatic or serious arrhythmias.
- Immunization: Immunize against influenza and Streptococcus pneumoniae.
- Surgical options: Consider left ventricular assist device (LVAD), heart transplant, volume reduction surgery, and implantable cardioverter-defibrillators (AICDs) for suitable candidates with severe, refractory disease.
Histopathological Features of Subtypes of Cardiomyopathies
- Abnormal Myofibrillary Arrangements: In cardiomyopathies, there is a disruption in the normal parallel arrangement of myofibrils. Instead, myofibrils are arranged irregularly, with side-to-side branch connections that are not typical.
- Loss of Myocytes: The loss of myocytes (heart muscle cells) leads to a multinucleated appearance in the tissue. This means that there are cells with multiple nuclei due to the fusion of myocytes or other reasons related to cell damage.
- Presence of Amyloid Deposits in RCM: In restrictive cardiomyopathy (RCM), there are deposits of amyloid material that appear pink under the microscope. These deposits are indicative of abnormal protein accumulation in the heart tissue.
Infective Endocrditis
Causative Organisms Associated with Infective Endocarditis
- Sub-acute Bacterial Endocarditis:Streptococcus viridans or enterococci are commonly associated with this condition.
- IV Drug Abusers / Right-sided Endocarditis:Staphylococcus aureus is often the causative organism.
- Left-sided Endocarditis:Staphylococcus aureus is more frequently implicated than enterococci.
- Prosthetic Valve Endocarditis:
- 2 months: Coagulase-negative staphylococci (C.O.N.S) are commonly found.
- 2-12 months: Coagulase-negative staphylococci (C.O.N.S) are still the main organisms.
- > 1 year:S. viridans becomes more common.
- Native Valve Endocarditis: This is usually community-acquired.
- Health Care-associated Endocarditis:
- Culture-negative Endocarditis:Granulicatella abiotrophia, Tropheryma whipplei, Coxiella, or Bartonella may be the causative organisms.
- Libman-Sacks Endocarditis: This condition is characterized by fibrin deposits on the inferior surface of the valves.
- Non-bacterial Thrombotic Endocarditis: This occurs due to endothelial damage leading to the formation of a platelet-fibrin thrombus.
Manifestations of Infective Endocarditis
- Involvement:
- Perivalvular abscess
- Congestive heart failure (CHF)
- Pericarditis
- Bundle branch block
- Myocardial infarction (MI)
- New onset murmur due to fistula in the valve
- The organisms in the deeper tissue layers are hard to remove and are not very active.
- Manifestations:
- Janeway lesions
- Osler nodes
- Splinter haemorrhage
- Mycotic aneurysms
- Kidney:
- Post-infectious glomerulonephritis often shows low C3 levels due to immune response consumption.
- Immunological manifestations:
- Roth spots
- Osler nodes
- Glomerulonephritis
- Causes of Roth Spots:
- Infective endocarditis
- Polyarteritis nodosa
- Leukaemia
- Roth's spots, which are retinal haemorrhages with a white centre, indicate infective endocarditis.
Major Blood Culture Criteria for Infective Endocarditis (IE)
- Two blood cultures positive for organisms typically associated with infective endocarditis (IE).
- Blood cultures are consistently positive for one of these organisms when taken more than 12 hours apart.
- Three or more separate blood cultures taken at least 1 hour apart.
Major Echocardiographic Criteria for IE
- An echocardiogram must show an oscillating intracardiac mass on a valve or supporting structures, documented in the path of regurgitant jets or on implanted material, with no other anatomical explanation.
- Myocardial abscess.
- Development of partial dehiscence of a prosthetic valve.
- New-onsetvalvular regurgitation.
Minor Criteria for Infective Endocarditis
- Heart condition or use of intravenous drugs
- Fever of 38°C (100.4°F) or higher
- Vascular issues, such as:
- Major arterial emboli
- Septic pulmonary infarcts
- Mycotic aneurysm
- Intracranial haemorrhage
- Conjunctival haemorrhage
- Janeway lesions
- Immunologic signs like:
- Glomerulonephritis
- Osler nodes
- Roth's spots
- Presence of rheumatoid factor
- Positive blood culture results not meeting major criteria or serological proof of active infection with an organism related to infective endocarditis (IE)
- Echocardiogram results indicating IE but not fulfilling major echocardiographic criteria
A definitive clinical diagnosis can be made based on:
- 1 major criterion and 3 minor criteria
- 5 minor criteria
Patients should be afebrile for less than 7 days. a fever lasting more than 7 days suggests a paravalvular abscess.
Treatment for Specific Organisms
Staphylococcus aureus (S. aureus)
- Treatment typically involves a combination of Nafcillin, Gentamicin, and Rifampicin for a duration of 8 weeks.
- Rifampicin is particularly effective in eliminating Staphylococcus infections associated with foreign material.
- For Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin should be added to the regimen.
HACEK Organisms
- Treatment options include:
- Ceftriaxone for 4 weeks
- Penicillin G with Gentamicin for 4 weeks
- For penicillin-sensitive Streptococcus bovis. Penicillin G with Ceftriaxone for 2 weeks
- For penicillin-resistant Streptococcus bovis. same treatment as above for 4 weeks
Indications for Surgery
- Aortic regurgitation leading to Congestive Heart Failure (CHF)
- Rupture of the sinus of Valsalva
- A persistent fever lasting more than 10 days with hypermobile vegetations may indicate a severe infection
Infective Endocarditis (IE) Prophylaxis
The American Heart Association (AHA) guidelines recommend IE prophylaxis only for specific groups:
- Patients with prosthetic valve material
- Individuals with a history of infective endocarditis
- Certain types of unrepaired congenital heart disease
- Cardiac transplant recipients who develop valvulopathy
Antibiotic prophylaxis is necessary only for certain procedures:
- Dental procedures
- Respiratory tract procedures
- Procedures on infected skin or skin structures
Prophylaxis is not required for gastrointestinal or genitourinary procedures.
Heart Sounds and Murmurs
Heart Sounds
- Atrial Systole
- Isovolumetric Contraction. S1 occurs )
- Ventricular Systole. ejection click )
- Isovolumetric Relaxation. S1 occurs )
- Ventricular Diastole. opening snap )
First Heart Sound S1 ( 25 - 45 Hz )
- S1 is the first heart sound, occurring when the MITRAL VALVE and TRICUSPID VALVE close.
- It is best heard using the diaphragm of the stethoscope.
S1 Loud
- Tachycardia
- Mitral Stenosis. MS )
- Physiological changes seen in pregnant women and children
S1 Soft
- Calcification of leaflet in MS
- Bradycardia
- Obesity, emphysema
- S1 has a lower frequency than S2.
Second Heart Sound S2 ( 50 Hz )
- S2 is the second heart sound, resulting from the closure of the AORTIC VALVE and PULMONIC VALVE.
- It is also heard with the diaphragm of the stethoscope.
Narrow Split
- Pulmonary atresia
- Tetralogy of Fallot ( TOF ). single S2 )
Wide Variable Split
- Mitral Regurgitation. MR )
- Ventricular Septal Defect. VSD )
- Right Bundle Branch Block. RBBB )
Wide Fixed Split
- Atrial Septal Defect. ASD )
- Pulmonary Arterial Hypertension. PAH )
- Pulmonary embolism
Loud S2
- Reversed Split
Conditions Related to Loud S2
- Hypertension. HTN )
- Ischemic Heart Disease. IHD )
- Aortic Stenosis. AS )
- Left Bundle Branch Block. LBBB )
- Aortic pulmonary shunt
Ejection Sound/Click
- Congenital bicuspid valve, aortic or pulmonary root dilation
- Soft sounds
- Calcified bicuspid aortic valve
- Non-ejection mid-systolic clicks
- Clicks. Mitral valve prolapse
- *The pulmonary ejection click is the only right-sided sound that becomes quieter with inspiration
Third Heart Sound S3 [Early Diastolic]
- The third heart sound, known as S3, is an early diastolic sound that occurs during the ventricular filling phase. It is characterized by its low frequency and is best heard using the bell of a stethoscope.
- S3 is commonly observed in children due to their higher cardiac output and more compliant ventricles. However, it can also be indicative of conditions such as dilated cardiomyopathy (DCM), congestive heart failure (CHF) , and constrictive pericarditis in adults.
Fourth Heart Sound S4 [Late Diastolic]
- The fourth heart sound, or S4, is produced during the atrial filling phase of the cardiac cycle and is best heard with the bell of a stethoscope. It is a low-frequency sound that can be associated with conditions such as aortic stenosis (AS) and pulmonary stenosis (PS) .
- In certain cases, additional sounds known as S1 and S2 may be present along with low-frequency sounds like the tumor plop. It's important to note that S1 may be weaker or absent in cases of atrial fibrillation.
- Normal heart sounds consist of S1, S2, S3, and S4, representing diastole and systole in phonocardiograms of both normal and abnormal heart sounds.
Diastolic Murmurs
Diastolic murmurs are abnormal sounds heard during the diastolic phase of the cardiac cycle, which is when the heart's ventricles relax and fill with blood. These murmurs can indicate various heart conditions, and they are classified based on their timing and characteristics.
Early Diastolic Murmurs
Mid Diastolic Murmurs
- Graham Steele murmur
- Carey Coombs murmur
- Aortic regurgitation (mild)
- Austin Flint murmur
- Pulmonic regurgitation (mild)
Flow murmurs are also considered a type of mid-diastolic murmur.
Carey Coombs is an example of a mid-diastolic murmur.
Causes of Aortic Regurgitation
- Valvular Causes:
- Congenital issues such as a bicuspid aortic valve
- Rheumatic endocarditis
- Valve prolapse
- Trauma to the valve
- Complications following valve surgery (post-valvulotomy)
- Dilatation of the Valve Annulus:
- Aortic dissection
- Annulo-aortic ectasia
- Cystic medial degeneration
- Hypertension
- Conditions like ankylosing spondylitis, syphilis, and Takayasu's arteritis
Causes of Pulmonary Regurgitation
- Valvular Causes:
- Post-valvulotomy complications
- Endocarditis
- Rheumatic fever
- Carcinoid syndrome affecting the valve
- Dilatation of the Valve Annulus:
- Pulmonary hypertension leading to annulus dilation
- Marfan's syndrome causing connective tissue abnormalities
- Takayasu's arteritis affecting the vascular system
- Congenital Causes:
- Isolated pulmonary regurgitation or associated conditions such as tetralogy of Fallot, ventricular septal defect (VSD), and pulmonic stenosis.
Systolic Murmurs
Systolic murmurs that occur during the ejection phase can be remembered using the mnemonic P.A.S.S.
- P.A.S.S stands for:
- Pulmonic Stenosis
- Aortic Stenosis
- Secondary to Secondary causes.
Pansystolic Murmurs
Pansystolic murmurs are associated with conditions such as:
- Pulmonic Stenosis
- Tricuspid Regurgitation
- Ventricular Septal Defect (VSD)
Mid-systolic clicks, which occur during mid-systole, are linked to mitral valve prolapse.
- Late Systolic Murmurs are observed in conditions like:
- Small Ventricular Septal Defect (VSD)
- Mitral Valve Prolapse
- Early Systolic Murmurs are associated with:
- Papillary Muscle Dysfunction
Continuous Murmurs
Continuous murmurs, commonly seen in patent ductus arteriosus, are characterised by various conditions including:
- Patent Ductus Arteriosus
- Aortopulmonary Window
- Coronary Arteriovenous Fistula
- Ruptured Sinus of Valsalva Aneurysm
- Cervical Venous Hum
- Anomalous Left Coronary Artery from the Pulmonary Artery
- Mammary Souffle of Pregnancy
- Bronchial Collateral Circulation
- Intercostal or Pulmonary Arteriovenous Fistula
- Peripheral Pulmonic Stenosis
Rheumatic Fever
(2015, Revised Jones criteria)
Major Criteria
- Low-risk populations
- Moderate- and high-risk populations
Carditis
- Clinical and/or subclinical
Arthritis
- Polyarthritis only
- Monoarthritis or polyarthritis
- Polyarthralgia
- Chorea
- Erythema marginatum
- Subcutaneous nodules
Low-risk populations
- Moderate- and high-risk populations
Monoarthralgia
- Fever (≥ 38.5°)
- Fever (≥ 38°C)
- ESR 20-30 mm/h and/or CRP. 3.0 mg/dL
- Prolonged PR interval, after accounting for age variability (unless carditis is a major criterion) in all populations.
Rheumatic Update
- The main change from older criteria is the use of echocardiography to diagnose subclinical carditis.
- The authors propose specific echocardiographic standards for diagnosing rheumatic valvular regurgitation of the mitral and aortic valves.
- In cases of rheumatic mitral valve prolapse, only the leaflet tip bends into the left atrium, which is different from the body of the valve leaflet in typical mitral valve prolapse.
- Another significant point is that monoarthritis and polyarthralgia are now considered major criteria in moderate to high-risk groups.
- Low-grade fever has also been added as a minor criterion in such populations.
- For diagnosing the recurrence of rheumatic activity, even three minor criteria without any major criteria can be considered.
- Polyarthralgia is a major criterion in moderate to high-risk groups but a minor criterion in low-risk populations.
Rheumatic Carditis
Symptoms and Findings
- Chest Pain at Rest: Individuals may experience chest pain even when at rest.
- Friction Rub:. notable sound indicating inflammation of the heart's outer layer.
- Congestive Heart Failure with Pulmonary Oedema: Fluid accumulation in the lungs due to heart failure.
- S3 Heart Sound: An additional heart sound often associated with heart failure.
- Carey Coombs Murmur:. characteristic heart murmur linked to rheumatic causes.
- Mitral Stenosis:. narrowing of the mitral valve, commonly associated with rheumatic carditis.
- Mitral Regurgitation in Active Carditis: Backflow of blood through the mitral valve during active inflammation.
- Least Common Valve Involvement - Pulmonic Valve: Involvement of the pulmonic valve is rare in rheumatic carditis.
- P Mitrale and P Pulmonale: Electrocardiogram findings indicative of pulmonary artery hypertension.
- Use of Steroids: Corticosteroids may be administered for treatment.
- Valvuloplasty for Acute Congestive Heart Failure:. surgical procedure to relieve symptoms by repairing the valve.
Medical Treatment for Acute Rheumatic Fever
Treatment for Group A Streptococcal Infection:
- Administer antibiotics for group A streptococcal infection, regardless of whether the bacteria is identified.
Pain and Inflammation Management:
- Use steroids and salicylates to alleviate pain and inflammation.
Heart Failure Management:
- Digitalis
- Fluid and sodium restriction
- Diuretics
- Oxygen
Management of Chorea in Acute Rheumatic Fever
Mild Cases:
- Create a calm environment for the patient.
Moderate Cases:
- Administer carbamazepine or valproate instead of haloperidol to manage symptoms.
Severe or Refractory Cases:
- Provide prophylaxis against Group A Beta-Hemolytic Streptococcus infections for patients with Acute Rheumatic Fever (ARF).
- Follow the recommendation of most authorities, including the American Heart Association, for prophylaxis for 5 years.
- Consider lifelong prophylaxis for individuals with rheumatic carditis.
AHA Guidelines for Duration of Secondary Prophylaxis
Rheumatic Fever without Carditis:
- Prophylaxis for 5 years after the last attack or until 21 years of age, whichever is longer.
Rheumatic Fever with Carditis but no Residual Valvular Disease:
- Prophylaxis for 10 years after the last attack or until 21 years of age, whichever is longer.
Rheumatic Fever with Echo-Proven Valvular Damage:
- Prophylaxis for 10 years after the last attack or until 40 years of age, whichever is longer.
Valvular Heart Disease
Comparison of Aortic Valve Diseases
- Aortic Stenosis
- Aortic Regurgitation
- Congenital Issues (bicuspid or unicuspid valves)
- Causes: Calcification, rheumatic disease
- Aortic Valve Area (AVA): 3-4 cm
- Severe Aortic Stenosis: 1.0 cm
- Critical Aortic Stenosis: <0.5 cm="">
- Supravalvular Aortic Stenosis
- Valvular Aortic Stenosis
- Acute Onset Conditions: Infective endocarditis, aortic dissection, trauma, failed prosthetic valve
- Symptoms: Exertional angina, syncope, dyspnea, paroxysmal nocturnal dyspnea (PND), orthopnea, peripheral edema
- Symptoms usually appear late in the disease, after left ventricular failure has developed.
- Common symptoms include dyspnea, orthopnea, PND, syncope, and angina.
Physical Examination Findings
Aortic Stenosis
- Pulse: Narrow pulse pressure, brachioradial delay, pulse parvus et tardus
- Auscultation: Crescendo-decrescendo systolic ejection murmur (SEM) radiating to the right clavicle, with a musical quality at the apex (Gallavardin phenomenon), soft S2 with paradoxical splitting, late S3
Aortic Regurgitation
- Auscultation: Early decrescendo diastolic murmur at the left lower sternal border (LLSB) (for cusp involvement) or right lower sternal border (RLSB) (for aortic root involvement), best heard when sitting, leaning forward, and on full expiration; soft S1, absent S2, late S3
Diagnostic Investigations
Aortic Stenosis
- ECG: Left ventricular hypertrophy (LVH) and strain, left bundle branch block (LBBB), left atrial enlargement (LAE), atrial fibrillation (AF)
- Chest X-ray (CXR): Post-stenotic aortic root dilatation, calcified valve, LVH, LAE, congestive heart failure (CHF)
- Echocardiogram (ECHO): Reduced valve area, pressure gradient, LVH, reduced left ventricular (LV) function
Aortic Regurgitation
- ECG: LVH, LAE
- CXR: LVH, LAE, aortic root dilatation
- Echocardiogram/Transesophageal echocardiogram (TTE): Quantification of aortic regurgitation (AR), identification of leaflet or aortic root anomalies
- Cardiac Catheterization (Cath): Considered for patients over 40 years old and surgical candidates to assess for ischemia
Aortic Stenosis Management
Asymptomatic Patients:
- Serial echocardiographic monitoring
- Avoidance of exertion
Symptomatic Patients:
- Avoidance of nitrates, arterial dilators, and angiotensin-converting enzyme inhibitors (ACEIs) in severe aortic stenosis
- Treatment of congestive heart failure (CHF) symptoms
- Surgical intervention if symptomatic or left ventricular dysfunction is present
Aortic Regurgitation Management
Medical Management:
- For aortic regurgitation with hypertension, angiotensin receptor blockers (ARBs) are preferred over beta blockers.
Surgical Indications:
- Symptomatic patients
- Asymptomatic patients with left ventricular end-diastolic pressure > 65 mm Hg
Surgical Options
Aortic Stenosis:
- Valve Replacement: Indicated for aortic rheumatic valve disease and trileaflet valves.
- Balloon Valvuloplasty: Considered in very young patients with aortic stenosis.
- High Gradient Severe Aortic Stenosis:
- Doppler jet velocity > 4 m/sec
- Mean gradient > 40 mm Hg
- Super Severe Aortic Stenosis:
- Doppler jet velocity > 5 m/sec
- Mean gradient > 55 mm Hg
- Asymptomatic patients with severe aortic stenosis and increased transvalvular gradient > 55 mm Hg require aortic valve replacement.
Aortic Regurgitation:
- Valve Replacement: For symptomatic patients and asymptomatic patients with elevated LV end-diastolic pressure.
- Valve Repair: Limited role, primarily for coarctation improvement.
- Bentall Procedure: Involves composite graft replacement of the aortic valve, aortic root, and ascending aorta, with re-implantation of the coronary arteries into the graft.
Comparison of Diseases of Mitral Valve
Mitral Stenosis
- Rheumatic disease is the most common cause.
- Congenital causes are rare.
- Severe mitral stenosis is indicated by a mitral valve area (MVA) of less than 1.2 cm².
- Congenital cleft leaflets can also cause stenosis.
- Left ventricular aneurysm and dilatation due to conditions like congestive heart failure, dilated cardiomyopathy, or myocarditis are associated with mitral stenosis.
- Infective endocarditis leading to abscess formation can cause stenosis.
- Hypertrophic obstructive cardiomyopathy, acute myocardial infarction, and conditions affecting the mitral valve such as myxoma, annulus calcification, chordae or papillary muscle issues, trauma, ischemia, or rupture can contribute to stenosis.
- Symptoms include dyspnea, orthopnea, fatigue, palpitations, peripheral edema, malar flush, and in severe cases, a pinched and blue appearance of the face.
Mitral Regurgitation
- Symptoms include dyspnea, paroxysmal nocturnal dyspnea, orthopnea, palpitations, and peripheral edema.
- A characteristic murmur is heard with mitral regurgitation.
Clinical Signs
- II S2
- Atrial fibrillation is indicated by the absence of the "a" wave on jugular venous pressure.
- Left parasternal lift
- Palpable diastolic thrill at the apex
Auscultation
- Mid diastolic rumble at the apex is best heard with the bell in the left lateral decubitus position following exertion.
- Loud S1 and opening snap following loud P2 are best heard during expiration.
- A long murmur and short A2-O5 interval correlate with worse mitral stenosis.
Investigation Findings
- ECG findings may show normal sinus rhythm or atrial fibrillation, left atrial enlargement, right ventricular hypertrophy, and right axis deviation.
- CXR may show left atrial enlargement, congestive heart failure, and mitral valve calcification.
- Echo/TTE may reveal restricted opening of the mitral valve.
- Patients over 40 years (males) or 50 years (females) may have a higher risk of concurrent coronary artery disease.
Management
- Avoid exertion and fever to prevent increased left atrial pressure.
- Treat atrial fibrillation and congestive heart failure.
- Optimize diastolic filling time using beta-blockers or digoxin.
Hemodynamics of Mitral Stenosis
- Mitral Stenosis causes higher pressure in the left atrium compared to the left ventricle during diastole, creating a pressure difference.
- This pressure gradient makes the mitral valve open and recoil faster, producing a loud S1 sound.
- Over time, if the valve becomes calcified, its recoil decreases, leading to a softer S1 sound.
Audible Sounds in Diastole
- During diastole, the stenotic mitral valve opens, resulting in an opening snap (OS), followed by a decrescendo murmur as blood flows through the stenosis.
- Contraction of the left atrium before S1 enhances the pressure difference, making the murmur louder just before S1.
- The severity of mitral stenosis (MS) is evaluated based on the murmur's length and its relationship to the gap between A2 and the opening snap.
- While murmur loudness does not definitively indicate severity, it can provide insights into the hemodynamic state.
- The murmur is a mid-diastolic type that intensifies just before systole.
- The Carey Coombs murmur, a mid-diastolic murmur, is associated with rheumatic myocarditis.
Pericardial Disease
Acute Pericarditis
Etiology of Pericarditis/Pericardial Effusion
- Idiopathic is the most common cause, usually thought to be viral.
- Viral causes include Coxsackie virus A, B (most common), and echovirus.
- Bacterial causes include S. pneumoniae and S. aureus.
- TB (tuberculosis).
- Fungal causes include Histoplasmosis and Blastomycosis.
- Mitral Regurgitationmay present with:
- Displaced, hyperdynamic apex.
- Left parasternal lift.
- Apical thrill.
- Holosystolic murmur at apex, radiating to axilla ± mid-diastolic rumble.
- Loud S2 (especially if pulmonary hypertension) and S3.
- ECG findings: Left atrial enlargement (LAE), left atrial delay (bifid P waves), ± left ventricular hypertrophy (LVH).
- CXR findings: LVH, LAE, pulmonary venous hypertension.
- Echo assesses severity of MR, left ventricular function, and leaflets.
- Swan-Ganz catheterisation. Prominent LA "v" wave.
- Asymptomatic. Serial echo monitoring recommended.
- Symptomatic. Manage by decreasing preload (diuretics) and afterload.
- ACE inhibitors may be used for severe MR in poor surgical candidates.
- Stabilise acute MR with vasodilators before surgery.
- Post-MI: Acute (direct extension of myocardial inflammation, 1-7 days) and Dressler's syndrome (autoimmune, 2-8 weeks) following cardiac surgery (e.g. CABG).
- Metabolic factors: Uremia (common), hypothyroidism.
- Neoplasms. Hodgkin's, breast cancer, lung cancer, renal cell cancer, melanoma.
- Collagen vascular diseases. SLE, polyarteritis nodosa, rheumatoid arthritis, scleroderma.
- Vascular causes: Dissecting aneurysm.
- Other factors. Drugs (e.g. hydralazine), radiation, infiltrative diseases (e.g. sarcoidosis).
Diagnostic triad. Chest pain, friction rub, and ECG changes.
- Pleuritic chest pain is often relieved by sitting up and leaning forward.
- Pericardial friction rub may be uni-, bi-, or triphasic.
- ± Fever and malaise.
- Initially, ST segment is diffusely elevated in all leads except aVR, ± depressed PR segment.
- The elevation in the ST segment is concave upwards.
- After 2-5 days, ST segment becomes isoelectric with T wave flattening and inversion.
- CXR. Normal heart size, may show pulmonary infiltrates.
- Echo. Used to assess pericardial effusion.
- Treat the underlying disease.
- Anti-inflammatory agents. High-dose NSAIDs/ASA; steroids if severe or recurrent; analgesics.
- Prognosis:
- Complications may include recurrence, atrial arrhythmia, pericardial effusion, tamponade, and constrictive pericarditis (uncommon).
- Transudative effusion (serous) may arise from CHF or hypoalbuminaemia.
- Exudative effusion (serosanguinous or bloody) has causes similar to acute pericarditis.
- Acute effusion may develop due to hemopericardium (e.g. trauma, post-MI myocardial rupture, aortic dissection).
Physiological effects depend on:
- Type and volume of effusion.
- Rate of effusion development.
- Underlying cardiac disease.
Symptoms may be:
- Asymptomatic or similar to acute pericarditis.
- Dyspnoea and cough due to involvement of nearby structures (e.g. recurrent laryngeal nerve, tracheobronchial, phrenic nerve irritation).
- JVP (jugular venous pressure) is increased with dominant "x" descent.
- Arterial pulse may be normal to decreased volume with a reduced pulse pressure.
- Auscultation may reveal distant heart sounds ± rub.
- ECG. Low voltage, flat T waves, and electrical alternans.
- CXR. Cardiomegaly and rounded cardiac contour.
- Echo. Procedure of choice for detecting fluid in the pericardial sac.
- Pericardiocentesis. Definitive method to distinguish transudate from exudate, identify infectious agents, and assess neoplastic involvement.
- Mild cases. Frequent observation with serial echocardiograms; treat the cause; anti-inflammatory agents for inflammation.
- Severe cases. Risk of cardiac tamponade.
Cardiac Tamponade
- Major complication of rapidly accumulating pericardial effusion; it is a clinical diagnosis.
- Causes of pericarditis include trauma, malignancy, uremia, idiopathic cases, and proximal aortic dissection with rupture.
- Not all large pericardial effusions lead to cardiac tamponade; it depends on the rate of development.
- In hypothyroidism, large effusions can develop slowly, similar to other serous cavity effusions.
- Symptoms include:
- Tachypnoea and dyspnoea.
- Shock.
- Pulsus paradoxus.
- JVP. "x" descent only, with absent "y" descent.
- Signs of hepatic congestion or peripheral oedema may occur.
- ECG. Electrical alternans (pathognomonic variation in R wave amplitude) and low voltage.
- Echo. Shows pericardial effusion and compression of cardiac chambers (right atrium and right ventricle) during diastole.
- Cardiac catheterisation may be performed.
- Pericardiocentesis. Can be done under echo or ECG guidance.
- Pericardiotomy may be necessary.
- Avoid diuretics and vasodilators, as these decrease venous return to an already under-filled heart.
- Fluid administration (e.g. saline load) may temporarily increase cardiac output.
Constrictive Pericarditis
- Chronic pericarditis leads to a thickened, fibrosed, adherent, and/or calcified pericardium.
- Any cause of acute pericarditis can result in chronic pericarditis.
- Main causes include:
- Idiopathic.
- Post-infectious (e.g. viral, TB).
- Radiation.
- Post-cardiac surgery.
- Uremia.
- Myocardial infarction.
- Common symptoms include:
- Dyspnoea.
- Fatigue.
- Palpitations.
Abdominal Pain
- Can mimic Congestive Heart Failure (CHF), particularly right-sided heart failure.
- Symptoms may include:
- Ascites (fluid accumulation in the abdomen)
- Hepatosplenomegaly (enlargement of the liver and spleen)
- Pedal edema (swelling of the feet and ankles)
- Signs may include:
- Elevated Jugular Venous Pressure (JVP)
- Kussmaul's sign (an increase in JVP during inhalation)
- Friedreich's sign (characterized by a prominent "y" descent in the JVP waveform)
- Blood pressure is typically normal, and pulsus paradoxus is not present.
- A pericardial knock (an early diastolic sound) may be observed.
ECG Findings
- The Electrocardiogram (ECG)may display non-specific abnormalities such as:
- Low voltage
- Flat T waves
- Possible occurrence of atrial fibrillation (AF)
- Chest X-ray (CXR). May reveal pericardial calcification and effusions.
Imaging and Diagnostics
- Echocardiography (Echo) /Computed Tomography (CT) /Magnetic Resonance Imaging (MRI). These imaging modalities can demonstrate pericardial thickening, with MRI also providing insights into myocardial involvement.
- Cardiac catheterization. This procedure can show equalization of end-diastolic chamber pressures, which is diagnostic for constrictive pericarditis.
Treatment Options
- Medical Management. Involves the use of diuretics and dietary salt restriction to manage symptoms.
- Surgical Intervention. Pericardiectomy (surgical removal of the pericardium) is considered only if medical management fails to provide relief.
Prognosis
- The prognosis is generally favorable for cases with idiopathic or infectious causes of constrictive pericarditis.
- Conversely, the outlook is poor for cases resulting from post-radiation damage, which often lead to progressive heart failure.
Differentiation between Constrictive Pericarditis and Cardiac Tamponade
- Cardiac Tamponade.
- Is a less common condition
- Characterized by the presence of a Pericardial Knock, which is always observed
Aortic Dissection
Aortic dissection occurs when there is a tear in the aortic intima, allowing blood to flow into the media. This condition can be classified as acute if it occurs within 2 weeks and chronic if it lasts longer than 2 weeks.
Classification
- De Bakey classification
- Type I: Involves both ascending and descending aorta
- Type II: Involves only the ascending aorta (stopping at the innominate artery)
- Type IIIA: Involves only the descending thoracic aorta (below the left subclavian artery and above the diaphragm)
- Type IIIB: Type IIIA plus involvement of the abdominal aorta
- Stanford classification
- Type A: Requires emergency surgery with cardiopulmonary bypass and may need hypothermic circulation for transverse arch dissections; involves the ascending aorta ± aortic arch and has a high initial mortality rate.
- Type B: Involves only the aorta below the subclavian artery; emergency surgery is needed only if complications arise (requires long-term follow-up to monitor aneurysm size).
The aortic media, which consists of smooth muscle and elastic tissue, is commonly damaged, leading to degenerative or cystic changes due to hypertension.
Other potential causes include:
- Cystic medial necrosis
- Atherosclerosis
- Connective tissue diseases (such as Marfan's and Ehlers-Danlos syndromes)
- Congenital conditions (such as coarctation of the aorta, bicuspid aortic valves, and patent ductus arteriosus)
- Infections
- Trauma
- Arteritis (such as Takayasu's arteritis)
Symptoms include:
- Sudden onset of tearing chest pain radiating to the back
- Hypertension in 75-85% of patients
- Asymmetric blood pressure and pulses between arms
- Ischemic syndromes due to blockage of aortic branches:
- Coronary (leading to myocardial infarction)
- Carotids (leading to ischemic stroke)
- Horner's syndrome
- Splanchnic (leading to ischemic gut)
- “Unseating” of aortic valve cusps (new diastolic murmur in 20-30% of cases)
- Rupture into pleura (causing dyspnoea and haemoptysis), peritoneum (resulting in hypotension and shock), or pericardium (leading to fluid accumulation around the heart affecting its function)
- Renal insufficiency
- Lower limb ischemia
CXR Findings
- Pleural cap (pleural effusion in lung apices)
- Widened mediastinum
- Left pleural effusion with blood extravasation
Investigations
- Transoesophageal echocardiogram (TEE): can visualise the aortic valve and thoracic aorta but not the abdominal aorta (preferred bedside investigation)
- Electrocardiogram (ECG): shows left ventricular hypertrophy, pericarditis, or heart block
- Cardiac MRI is preferred for stable patients (shows a "tennis ball appearance")
- Troponin I test to rule out myocardial infarction
Medical Treatment
- Esmolol or labetalol to lower blood pressure and reduce cardiac contractility
- Target systolic blood pressure of 110 mm Hg and heart rate of 60 bpm
Surgical Treatment
- Resection of the intimal tear and restoration of flow through the true lumen
- Replacement of the affected aorta with a prosthetic graft
- Correction of any predisposing factors (e.g., bicuspid aortic valve, patent ductus arteriosus)
- Type A: Requires emergency surgery with cardiopulmonary bypass; initial mortality rate increases rapidly without surgery.
- Type B: Initially managed with medication; 10-20% may require urgent surgery for complications (such as expansion, rupture, or compromised branch arteries).
SYNCOPE
Syncope is a sudden and temporary loss of consciousness due to reduced blood flow to the brain. If a patient requires CPR or cardioversion, it is not syncope. The main causes of syncope are neurocardiogenic (vasovagal) and cardiovascular reasons.
In neurocardiogenic syncope, increased sympathetic activity leads to stronger contractions of the left ventricle (LV), stimulating mechanoreceptors in the LV and causing heightened vagal activity through the Bezold-Jarisch reflex. Actions such as coughing, swallowing, passing stools, and urination can increase vagal tone.
Orthostatic Hypotension:
- Hypovolemia or diuretic use
- Autonomic neuropathy
- Parkinsonism
- Multiple system atrophy
- Lewy body dementia
- Postural orthostatic tachycardia syndrome (dysautonomia)
Cardiovascular:
- Bradyarrhythmias: Sick sinus syndrome, complete heart block
- Tachyarrhythmias: VT, SVT, Wolff-Parkinson-White syndrome
- Valvular disease: Aortic stenosis, atrial myxoma
- Others: Cardiac tamponade, hypertrophic obstructive cardiomyopathy
- Vertebrobasilar insufficiency
- Subarachnoid hemorrhage (SAH)
- Transient ischemic attack (TIA)/Stroke
- Migraine
- Miscellaneous causes: hypoglycemia, narcolepsy, psychological reasons
Temporary convulsions may occur due to brain oxygen deprivation and can resemble seizures. Patients with vasovagal syncope do not have a higher risk of death, myocardial infarction (MI), or stroke compared to the general population.
Hypertension
Systolic mm Hg
- Pre-hypertension: 120-139
- Stage I hypertension: 140-159
- Stage II hypertension: ≥160
- Isolated systolic: ≥140
Diastolic mm Hg
- Pre-hypertension: 80-89
- Stage I hypertension: 90-99
- Stage II hypertension: ≥100
Blood Pressure Monitoring
- Average 24-hour blood pressure: ≤135/85 mm Hg
- Asleep BP: >120/75 mm Hg
- Approximate clinic BP value: 140/90 mm Hg
Causes of Hypertension
- The most common congenital cause is coarctation of the aorta.
- Heart disease is the leading cause of death in patients with severe hypertension or underlying conditions.
Hypertension Update 2017
Categories of Blood Pressure in Adults
Blood pressure (BP) is categorized based on systolic blood pressure (SBP) and diastolic blood pressure (DBP) readings. Here are the categories:
- Systolic Blood Pressure (SBP). < 120="" mm="" hg="">
- Normal. 120-129 mm Hg
- Stage 1 Hypertension. 130-139 mm Hg
- Stage 2 Hypertension. ≥140 mm Hg
Diastolic Blood Pressure (DBP). < 80="" mm="" hg="">
- Stage 1 Hypertension. 80-89 mm Hg
Note: Readings should be the average of more than two careful measurements taken on more than two occasions.
Causes of Secondary Hypertension
- Resistant Hypertension: High blood pressure that does not respond to standard treatments.
- Poor Sleep Quality: Inadequate or disrupted sleep can contribute to hypertension.
- Overnight Oximetry Issues: Problems detected during overnight oxygen monitoring may be linked to hypertension.
- Renovascular Disease: Conditions affecting the blood vessels in the kidneys can lead to high blood pressure.
- Flash Pulmonary Oedema: Sudden accumulation of fluid in the lungs can be associated with hypertension.
- Fibromuscular Dysplasia:. condition affecting blood vessels, particularly in women, can cause early-onset hypertension.
- Renal Doppler Concerns: Issues identified through renal Doppler imaging may indicate underlying causes of hypertension.
- Primary Aldosteronism: An endocrine disorder leading to excessive production of aldosterone, contributing to hypertension.
- Hypertension with Hypokalaemia: High blood pressure accompanied by low potassium levels may indicate specific causes.
- Plasma Aldosterone: Renin Ratio Tests: Diagnostic tests to assess aldosterone and renin levels in relation to each other.
- Drug Abuse: Use of substances like cocaine, ephedrine, and amphetamines, as well as smoking (nicotine), can lead to hypertension.
- Symptoms of Drug Abuse: Fine tremors, rapid heartbeat (tachycardia), and chest pain may indicate drug abuse contributing to hypertension.
- Urinary Drug Screening: Testing urine for drugs can help identify potential causes of hypertension related to substance abuse.
- Renal Parenchymal Disease: Disorders affecting the kidney tissue can be a cause of secondary hypertension.
- Urinary Tract Infection (UTI) and Analgesic Abuse: UTIs and misuse of pain relievers may be linked to hypertension in some cases.
- Polycystic Kidney Disease:. genetic disorder characterized by cysts in the kidneys can lead to hypertension.
- Renal Ultrasound Findings: Abnormalities detected in renal ultrasound imaging may indicate underlying causes of hypertension.
Blood Pressure Goals for Pharmacological Treatment in Hypertensive Patients
- Clinical Conditions
- Blood Pressure (BP) Goal (mmHg)
- Clinical Cardiovascular Disease (CVD) or 10-year Atherosclerotic Cardiovascular Disease (ASCVD) Risk > 10%
- Chronic Kidney Disease /Post-Transplantation
- Heart Failure /Stable Ischemic Heart Disease
- Secondary Stroke Prevention
Management of Hypertensive Crisis
- If systolic blood pressure (sBP) is greater than 180 mm Hg and/or diastolic blood pressure (DBP) is greater than 120 mm Hg, evaluate for potential new or worsening target organ damage.
- In cases of hypertensive emergency, admit the patient to the Intensive Care Unit (ICU) and assess for conditions such as aortic dissection, eclampsia, or pheochromocytoma crisis.
- Aim to reduce blood pressure by a maximum of 25% within the first 2 hours, followed by a target range of 160/100-110 mm Hg over the subsequent 24 hours.
- If there is no significant elevation in blood pressure, consider intensifying oral treatment.
Unequal Blood Pressure between Left and Right Arm
- Coarctation of the aorta
- Supravalvular aortic stenosis
- Obstructive aortoarteritis
The Coanda effect describes how a jet stream adheres to the right wall of the aorta, resulting in higher systolic blood pressure (BP) in the right arm. This effect is seen in supravalvular aortic stenosis. The murmur associated with supravalvular aortic stenosis (S.V.A.S) is an ejection systolic murmur.
- SBP in the upper limbs is greater than SBP in the lower limb, which is typical of postductal coarctation of the aorta.
- SBP in the lower limbs exceeds SBP in the upper limbs by more than 20 mmHg, which is seen in valvular aortic regurgitation.
Hypertension with Metabolic Alkalosis (Liddle Syndrome)
- Inherited condition that is passed down in an autosomal dominant manner.
- Enhanced activity of the epithelial sodium channel known as ENaC.
- This leads to an increase in plasma volume and salt retention, causing hypertension.
- There is a corresponding loss of potassium and hydrogen ions in the urine, resulting in hypokalemic alkalosis.
- The medication Amiloride is used to block ENaC and manage this condition.
Pseudohypoaldosteronism is a related condition that we will now discuss.
Hypertension with Metabolic Acidosis ( Pseudohypoaldosteronism )
- Renal Tubular Insensitivity or Resistance to the action of Aldosterone
- Further subclassified as:
- PHA Type 1. the classic form
- PHA Type 2. Gordon syndrome / Chloride shunt syndrome
- PHA Type 2. Gordon syndrome is an autosomal dominant disorder due to mutations in genes encoding WNK kinases
- Sustained volume depletion triggers hyperreninism and hyperaldosteronism, leading to hypertension
- Increased chloride reabsorption in the distal nephron enhances sodium reabsorption, affecting the mineralocorticoid-dependent voltage
- This limited voltage gradient reduces the driving force for potassium and hydrogen ions.
Preferred Parenteral Medications for Specific Hypertensive Emergencies
- NicardipineMalignant Hypertension
- Acute Left Ventricular Failure (LVF)Nitroprusside, EsmololAdrenergic Crisis
- PhentolamineHypertensive Encephalopathy
Management of Hypertension
- For individuals under 55 years old, initiate treatment with A, C, and D.
- In cases of resistant hypertension (often due to non-compliance), start with A, C, D, and consider adding spironolactone or an alpha/beta-blocker.
- A. ACE/ARB Inhibitor; C. Calcium Channel Blocker; D. Diuretic
- Prevalence is 6 - 8 per 1000 live births (specific condition not mentioned).
- Inheritance is multifactorial.
Criteria for Diagnosis of Congenital Heart Disease
- Abnormal S2
- Systolic murmur of grade 3 or more accompanied by a thrill
- Systolic murmur of grade less than 3
- Diastolic murmur
- Cyanosis
- Abnormal ECG
- Abnormal X-ray
- Abnormal BP
Diagnosis of Congenital Heart Disease
The presence of one major criterion or two minor criteria is necessary to indicate the presence of heart disease.
Prenatal Risk Factors for CHD
Intrauterine infections, particularly Rubella, which is associated with conditions such as Patent Ductus Arteriosus (PDA), pulmonary stenosis, and Ventricular Septal Defect (VSD)
- Gestational diabetes, which is linked to VSD, PDA, and cardiomyopathy
- Maternal lupus, which can lead to complete heart block in the fetus
- The use of teratogenic drugs during the first trimester, including anticonvulsants, thalidomide, and retinoic acid, which can disrupt normal fetal development
- Phenylketonuria in the mother, which is associated with a higher risk of VSD, Tetralogy of Fallot (TOF), and PDA in the offspring
Classification of Congenital Heart Disease (CHD)
Congenital Heart Disease (CHD) can be classified into various categories based on the specific characteristics of the heart defects. Here are the main classifications:
1. Acyanotic Heart Defects
2. Cyanotic Heart Defects
3. Left-to-Right Shunt
Atrioventricular Septal Defect (AVSD), also known as Endocardial Cushion Defects, is a type of congenital heart defect where there is a deficiency in the septum that divides the heart's chambers. This defect allows blood to flow from the left side of the heart to the right side, leading to increased blood flow to the lungs.
4. Obstructive Lesions
Mitral and Tricuspid Valve Regurgitation are conditions where the heart's mitral and tricuspid valves do not close properly, allowing blood to flow backward into the heart chambers. This can lead to increased pressure and volume in the heart and can be classified as an obstructive lesion in congenital heart disease.
5. Decreased Pulmonary Blood Flow
Pulmonary Atresia/Critical Pulmonary Stenosis, Tetralogy of Fallot (TOF), and Tricuspid Atresia are conditions characterized by restricted blood flow to the lungs. In these conditions, the pulmonary artery or valve is obstructed or underdeveloped, leading to decreased oxygenation of blood and cyanosis.
6. Increased Pulmonary Blood Flow
Transposition of Great Arteries (TGA), Truncus Arteriosus, and Total Anomalous Pulmonary Venous Connection (TAPVC) are conditions where there is an abnormal connection or position of the major blood vessels leading from the heart. These defects result in increased blood flow to the lungs and can cause heart failure if not treated.
7. Special Variety
Pulmonary Vascular Obstructive Disease, also known as Eisenmenger Physiology, is a condition where there is high blood pressure in the pulmonary arteries due to obstruction. This leads to a reversal of blood flow through the shunt, causing cyanosis and other complications.
Ventricular Septal Defect (VSD)
VSD is the most common type of congenital heart disease (CHD).
Types of Ventricular Septal Defect
- Infundibular VSD: Also called subarterial or supracristal VSD.
- Perimembranous VSD: This is the most common subtype of VSD.
- Inlet VSD: This type is usually associated with endocardial cushion defects.
- Muscular VSD: This is the second most common type of VSD and has a high chance of spontaneous closure.
Symptoms of VSD typically appear at 6 to 8 weeks of age, with large VSDs leading to congestive cardiac failure (CCF) at the onset.
Examination Findings
- A loud pansystolic murmur at the lower left sternal border is a key indicator of VSD.
- In cases of small left-to-right shunts, S3 heart sound may be present.
X-ray Chest Findings
- Cardiomegaly: This depends on the size of the VSD shunt.
- Small VSDs: These typically show a normal heart shadow on X-ray.
- Left axis deviation: This finding suggests left atrial and ventricular hypertrophy.
Complications
- Symptoms of congestive cardiac failure (CCF)
- Recurrent chest infections
- Infective endocarditis: VSD is the most common congenital lesion affected by this condition.
- Eisenmenger syndrome: This involves the development of pulmonary arterial hypertension and the reversal of shunt.
- Assessing the chance of spontaneous closure
- Treating any anemia and other complications
Surgical Treatment
- Patch closure: This is the preferred treatment for VSD.
- Catheter closure: This option is suitable for children over 8 years old with muscular VSD.
Indications for Surgery
- Early onset of congestive cardiac failure (CCF) in infancy.
- Qp: Qs ratio greater than 2:1, indicating that pulmonary blood flow is more than twice that of systemic flow, which signifies a large shunt.
- Presence of related issues such as pulmonary stenosis.
Atrial Septal Defect
Atrial Septal Defect (ASD) is the most prevalent type of congenital heart defect (CHD) found in adults. It involves a defect in the atrial septum, which is the wall separating the left and right atria of the heart.
Anatomical Types of Atrial Septal Defect
- Ostium secundum ASD (fossa ovalis defect) is the most common type and is located in the central part of the atrial septum.
- Ostium primum ASD is found in the lower part of the atrial septum and is associated with endocardial cushion defects.
- Sinus venosus ASD occurs at the junction of the superior vena cava and right atrium.
- Coronary sinus ASD involves a defect in the atrial septum near the coronary sinus.
Auscultatory Findings
- The characteristic "machinery" murmur is heard continuously, best in the second left intercostal space, near the sternal border and below the left clavicle. This murmur results from blood flow across the ductus arteriosus during both systole and diastole.
- Patients may exhibit a large volume pulse and a wide pulse pressure.
- S1 is typically accentuated, while S2 may show paradoxical splitting in cases of large PDA.
- S3 is indicative of a large shunt.
Complications
- The complications associated with ASD are similar to those seen in Ventricular Septal Defect (VSD).
- In preterm babies, prostaglandin inhibitors such as Indomethacin or ibuprofen may be beneficial if started before the age of 2 weeks.
- For term babies, surgical ligation or catheter-based coil closure of the defect is recommended.
Causes of Continuous Murmur in Childhood
- Patent ductus arteriosus (PDA) is commonly associated with a continuous murmur heard in the second left intercostal space.
- Rupture of the sinus of Valsalva (RSOV) can also produce a continuous murmur.
- Other causes include aortopulmonary window (AP Window), anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), surgical shunts such as the Blalock-Taussig shunt in Tetralogy of Fallot (TOF), bronchopulmonary collaterals seen in TOF, tricuspid atresia, and truncus arteriosus, peripheral pulmonary artery stenosis, pulmonary and coronary arteriovenous (AV) fistulas, intercostal AV fistulas, collaterals in coarctation of the aorta (heard over the thoracic spine), and venous hum and mammary souffle.
Auscultatory Findings in Atrial Septal Defect
- A widely split and fixed S2 is a characteristic finding in patients with ASD.
- Unlike in VSD, there is not much pressure difference between the left and right atria, so there is no shunt murmur across the defect.
- Electrocardiogram (ECG) findings in ASD typically show right axis deviation, with the characteristic rsR' pattern seen in over 90% of patients. However, ostium primum ASD is an exception and is associated with left axis deviation.
- Right atrial and right ventricular enlargement is common in ASD.
Complications and Management
- Complications similar to those seen in VSD can occur at any age, but they are more common in adulthood.
- Closure of ASD is recommended to prevent complications such as atrial arrhythmias and heart failure in late adulthood.
- Transcatheter closure using an occluder device is usually the preferred method for ASD closure.
Patent Ductus Arteriosus (PDA)
PDA is a condition where the ductus arteriosus, a blood vessel that connects the pulmonary artery and the aorta, does not close as it should after birth. This condition is more common in females, with a ratio of 2:1, and is often associated with congenital rubella infection during pregnancy. If the PDA is significant, it can lead to congestive cardiac failure (CCF) in infants around 6 to 8 weeks of age.
Assessing the Probability of Spontaneous Closure of Heart Defects
Likelihood of Spontaneous Closure
- PDA in a term baby is unlikely to close on its own.
- PDA in a preterm baby has a high chance of spontaneous closure.
- Atrial septal defect (ASD) or ventricular septal defect (VSD) persisting beyond the age of 3 years is unlikely to close spontaneously.
Location of Defects
- VSD:
- Muscular VSD has a high likelihood of spontaneous closure.
- Inlet VSD and malaligned VSD (as seen in Tetralogy of Fallot) are very unlikely to close spontaneously.
- ASD:
- Ostium secundum ASD has a high likelihood of spontaneous closure.
Size of Defects
- ASD greater than 8 mm in size and large, unrestrictive ventricular septal openings (VSOs) are unlikely to close spontaneously.
Eisenmenger Syndrome
Eisenmenger syndrome is a condition that arises from a combination of several factors related to heart and lung physiology. Initially, it involves a left-to-right shunt, which leads to increased pulmonary blood flow. Over time, this increased blood flow causes vascular remodeling and endothelial dysfunction in the pulmonary vasculature.
As a result of these changes, pulmonary vascular resistance increases, leading to right ventricular hypertrophy (RVH). Eventually, the condition can progress to a right-to-left shunt, which is a critical and serious development in the syndrome.
Cyanosis
- In the early stages of Eisenmenger syndrome, there may be a paradoxical improvement in congestive symptoms, which can be misleading. However, as the condition progresses, cyanosis (a bluish discoloration of the skin due to lack of oxygen) becomes evident.
- Other clinical features that may be observed include clubbing (enlargement of the fingers or toes due to chronic low oxygen levels) and a parasternal heave (an abnormal movement of the chest wall near the sternum due to right ventricular enlargement).
Chest X-ray:
- Normal heart size. In cases of Eisenmenger syndrome, the heart size on a chest X-ray may appear normal, which can be misleading.
- Plethora of hilar regions with oligemia in peripheral lung fields. This refers to the abnormal distribution of blood vessels in the lungs seen on X-ray. There is increased blood flow (plethora) in the hilar regions (the area where the bronchi enter the lungs) and decreased blood flow (oligemia) in the peripheral lung fields. This finding is indicative of the vascular changes associated with Eisenmenger syndrome.
Treatment:
- Heart-lung transplantation is often the most definitive treatment for Eisenmenger syndrome, especially in advanced cases where there is severe pulmonary vascular disease and right heart failure.
- In some instances, primary surgical correction of congenital defects such as ventricular septal defect (VSD), atrial septal defect (ASD), or patent ductus arteriosus (PDA) may be considered. However, this approach may be contraindicated depending on the specific clinical scenario and the extent of pulmonary vascular disease.
Tetralogy of Fallot (TOF)
TOF is a type of cyanotic heart disease that can be managed without surgery after infancy.
The classic set of four features includes:
- Ventricular Septal Defect (VSD)
- Overriding aorta
- Subpulmonic infundibular stenosis (the main factor causing cyanosis)
- Right ventricular hypertrophy (concentric type)
The main symptom is a gradual worsening of cyanosis that appears after birth.
The level of cyanosis is related to how severe the pulmonic stenosis is, but the loudness of the systolic murmur is lessened when pulmonic stenosis is more severe.
Congestive heart failure (CCF) can happen in TOF cases, especially during stressful situations or with other issues present.
A loud, single S2 sound is noted (this is A2, as the pulmonic part is delayed and too quiet to hear).
No murmur from the shunt (VSD) is heard because:
- The VSD is large and allows blood to flow freely.
- The pressures in both ventricles are similar, resulting in little or no left-to-right shunt, making the VSD silent.
Chest X-ray
- 'Boot' shaped heart or 'Coeur en Sabot' appearance
- Normal size heart with an upturned apex (due to RVH)
- Concave pulmonary artery segment gives the heart its characteristic boot shape
Possible complications include:
- Infective endocarditis
- Neurological complications
- Cyanotic spells
Tetralogy of Fallot includes ASD, RVH, and PS.
Pentalogy of Fallot: TOF + ASD.
Cyanotic Spells (also known as "let" spells, Anoxic, Hypoxic, or Hypercyanotic Spells)
Age group:. months - 2 years
Precipitating factors: vigorous crying, exertion
- At the onset of a cyanotic spell, there is a decrease in systemic vascular resistance (SVR) and spasm of the infundibulum, which are crucial events.
Mechanism of Cyanotic Spell
- Spasm of the right ventricular outflow tract (RVOT) increases systemic vascular resistance (SVR), leading to left-to-right shunting during crying.
- Increased systemic venous return, hyperpnea, increased pO2, decreased pCO2, and decreased pH are observed.
- During a spell, increasing cyanosis and decreased intensity of the heart murmur are noted.
- If a cyanotic spell is not treated promptly, serious consequences like limpness, convulsions, stroke, or even death may occur.
Management of Cyanotic Spells
- Knee-chest position: This position helps increase blood flow to the lungs and improve oxygenation.
- Morphine: Administered to alleviate breathing difficulties during a cyanotic spell.
- Sodium bicarbonate: Used to correct metabolic acidosis that may occur during the spell.
Shunt Procedures in Tetralogy of Fallot (TOF)
- Right and Left pulmonary arteries: Shunt procedures may involve these arteries to improve blood flow to the lungs.
- O, inhalation: This likely refers to the use of inhaled oxygen to support patients.
- Beta blockers: These medications are used to manage infundibular spasm,. condition that can worsen TOF symptoms.
- Alpha agonists: These drugs help increase systemic vascular resistance, which can be beneficial in certain cases of TOF.
Prevention of Tetralogy of Fallot Complications
- Correction of related anaemia: Addressing any associated anaemia is crucial for improving overall health and reducing the risk of complications.
- Palliative surgical (shunt) procedures: These procedures aim to enhance pulmonary blood flow in infants with severe hypoxic spells who are not candidates for immediate corrective surgery.
- Blalock-Taussig (B-T) shunt: This procedure involves creating a connection between the subclavian artery and the same side pulmonary artery to improve blood flow to the lungs.
- Gore-Tex Interposition shunt (modified B-T shunt): Similar to the B-T shunt, this procedure uses Gore-Tex material to create a connection between the subclavian artery and the same side pulmonary artery.
- Waterston shunt: This shunt connects the ascending aorta to the right pulmonary artery, enhancing blood flow to the lungs.
- Potts shunt: This procedure involves connecting the descending aorta to the left pulmonary artery to improve pulmonary blood flow.
Transposition of Great Arteries (TGA)
Types of TGA
- Complete Type. In this type, the aorta arises from the right ventricle, while the pulmonary artery originates from the left ventricle.
- Physiologically Corrected Transposition. Here, the right atrium connects to a morphologically altered left ventricle, which is linked to the pulmonary artery. Simultaneously, the left atrium connects to a morphologically altered right ventricle, which leads to the aorta. Despite this anatomical reversal, the blood flow remains normal, which is why it is still classified as TGA.
The complete type is also referred to as D-TGA because, in this condition, the aorta is positioned in front of and to the right of the pulmonary artery.
Survival and Symptoms
- Survival in TGA depends on the presence of connections such as Ventricular Septal Defect (VSD), Atrial Septal Defect (ASD), or Patent Ductus Arteriosus (PDA), due to the separation of systemic and pulmonary blood flows.
- TGA with intact ventricular septum. This condition results in cyanosis at birth and leads to congestive heart failure within the first week of life.
- TGA with VSD. This variant causes mild cyanosis and congestive heart failure to occur 6-8 weeks after birth. A single S2 sound is a notable finding during auscultation in this case.
Chest X-ray Appearance
On a chest X-ray, TGA typically presents with an 'Egg on side' or 'Egg on string' appearance. This indicates cardiomegaly with a narrow base and enlarged lung fields.
|
16 docs|47 tests
|
FAQs on Cardiology Chapter Notes - Medicine - NEET PG
| 1. What is the significance of detecting tachyarrhythmias and bradyarrhythmias on an ECG? |  |
| 2. What are the causes of right axis deviation on an ECG? |  |
| 3. How can left axis deviation be interpreted on an ECG, and what are its potential causes? |  |
| 4. What are the distinguishing features of left bundle branch block (LBBB) and right bundle branch block (RBBB) on an ECG? |  |
| 5. What are the ECG findings associated with myocardial ischemia and infarction? |  |















