Endocrinology- 1 Chapter Notes | Medicine - NEET PG PDF Download
| Table of contents |

|
| Pituitary Disorders |

|
| Diabetes Mellitus |

|
| Long-Term Complications of Diabetes |

|
| Metabolic Syndrome |

|
| Adrenal Gland |

|
Pituitary Disorders
- Trophic hormone failure happens when the pituitary gland is either compressed or destroyed. The first hormone to decrease is growth hormone, followed by FSH, LH, TSH, and ACTH.
- In adults, the most common sign of hypopituitarism is hypogonadism, while in children, it is growth retardation.
- Pituitary apoplexy refers to bleeding within the pituitary gland, often occurring in conjunction with pre-existing adenomas or conditions such as diabetes, hypertension, and sickle cell anaemia. This condition represents a serious endocrine emergency that can lead to severe hypoglycaemia, hypotension, and shock.
- Urgent surgical decompression can be employed to alleviate symptoms such as: Progressive visual loss Cranial nerve palsy Loss of consciousness
- Empty sella is an incidental finding observed on MRI scans, associated with intracranial hypertension. Patients typically exhibit normal pituitary function, indicating that the surrounding pituitary tissue remains functional.
Kallman Syndrome
- Defective hypothalamic gonadotropin-releasing hormone (GnRH)
- Anosmia or hyposmia due to olfactory bulb agenesis
- Colour blindness, optic atrophy
- CNS abnormalities: Mirror movements
- Males: Delayed puberty, micropenis, and low testosterone
- Females: Primary amenorrhea
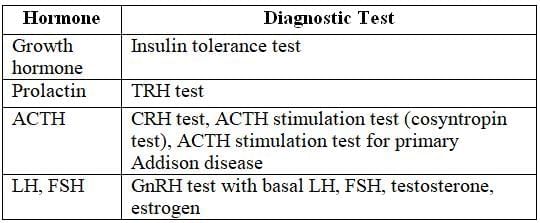
Tests of Pituitary Sufficiency
Familial Pituitary Tumor Syndromes
- MEN1 (11q13)
Pituitary Adenoma, Hyperparathyroidism, Zollinger-Ellison Syndrome, Foregut Carcinoids - MEN4 (12p13)
Pituitary Adenoma, Hyperparathyroidism, Reproductive Organ Tumours - Carney Complex (17q23-24)
Pituitary Hyperplasia, Atrial Myxoma
The most common type of pituitary tumour is a non-functioning or null cell tumour. In some cases, these tumours may produce gonadotropins.
Elevated levels of prolactin can occur in various situations, including:
- Postictal period (after a seizure)
- Primary hypothyroidism
- Chronic renal failure
Prolactinoma
Prolactinoma is a type of tumour that originates from lactotrope cells in the pituitary gland. These tumours account for approximately 50% of all functioning pituitary tumours. When prolactinomas grow larger than 1 cm, they are referred to as macroadenomas. These larger tumours can exert pressure on surrounding structures in the brain, leading to complications such as bitemporal hemianopia, a condition characterized by loss of vision in the outer (temporal) fields of both eyes.
In general, prolactin levels exceeding 250 µg/L are associated with the presence of macroadenomas.
Clinical Features
- Women might experience amenorrhea (missing periods), infertility, and galactorrhea (unexpected milk production).
- They could also suffer from headaches and visual problems.
- Men usually show larger tumors because signs of hypogonadism in males are less obvious.
Investigations
- MRI of the head is commonly performed.
- Serum prolactin levels are checked; values below 100 µg/l usually indicate microadenomas or sellar lesions that lower prolactin levels.
Treatment
- Treatment is not required if patients are asymptomatic and do not plan to conceive, as microadenomas seldom progress to macroadenomas.
- Cabergoline is an effective, long-lasting dopamine agonist that can significantly lower prolactin levels for more than 14 days after a single dose and often leads to the reduction of prolactinomas in most cases.
- Bromocriptine is prescribed for hyperprolactinaemia, particularly when pregnancy is intended, and it is safe for the fetus.
Acromegaly
Acromegaly is primarily caused by a GH -producing somatotroph adenoma. Other causes include mixed mammosomatotropic adenomas that produce both GH and prolactin. An increase in GH levels can also be due to a carcinoid tumor producing GHRH. In rare cases, an extra-pituitary source like a pancreatic islet cell tumor may be involved.
Symptoms of Acromegaly
- Frontal bossing: Enlargement of the forehead.
- Increase in the size of hands and feet, leading to spade-like hands.
- Prognathism: Protrusion of the jaw with widened spaces between lower incisors.
- Frequent changes in shoe size, glove size, and tightening of rings.
- Heel pad thickness: Increased thickness of heel pads.
- Coarse facial features accompanied by hyperhidrosis (excessive sweating) and acanthosis nigricans (dark patches of skin).
- Development of hypertension, which can lead to left ventricular hypertrophy (LVH) and ischaemic cardiomyopathy.
- Impaired glucose tolerance or diabetes mellitus.
- Joint problems (arthropathy) and muscle weakness.
- Visual field defects due to compression of the optic chiasma.
- Increased risk of developing colonic polyps.
 Spade like hand
Spade like hand
Workup
- The initial screening test involves measuring IGF-1 levels.
- The gold standard test is the glucose suppression test, which indicates a failure to suppress GH levels below 0.4 µg after an oral glucose challenge.
- There is often an increase in prolactin levels and a decrease in TSH production due to the mass effect of the tumor.
- Transsphenoidal surgery is the preferred treatment for resecting the macroadenoma.
- The earliest postoperative responses include:
- Decreased soft tissue swelling.
- Reduction in GH hormone levels within 1 hour.
- Decrease in IGF-1 levels within 3 to 4 hours.
- Somatostatin analogues, such as Lanreotide, Octreotide, and Pasireotide, are administered subcutaneously to reduce tumor size by targeting SSTR2 and SSTR5 receptors, thereby decreasing GH production.
- Pegvisomant is used to antagonize the effects of GH at the receptor level, although it does not directly affect the pituitary adenoma.
Diabetes Mellitus
Causes of Type 1 Diabetes Mellitus
- Autoimmunity: Involves specific genetic markers such as HLA DQ-A1* 0301, DR-3, and DR-4. The CTLA-4 gene plays a crucial role in regulating insulin production.
- Viral Damage: Infections by viruses like Coxsackie-B, mumps, and rubella can cause significant damage leading to diabetes.
- Bronzediabetes: Form of diabetes associated with certain conditions.
- Latent Autoimmune Diabetes in Adults: Also known as Type 1.5 diabetes mellitus, this condition is triggered by the anti-GAD autoantibody, which damages beta cells in the pancreas.
Reclassification of Type 1 Diabetes Mellitus:
- Type 1A: Diabetes caused by a combination of genetic and environmental factors.
- Type 1B: Idiopathic cases of Type 1 diabetes with no known cause.
Diagnostic Markers in Type 1 Diabetes Mellitus
- Insulin Autoantibodies (IAA): Present in 40-70% of cases.
- Glutamic Acid Decarboxylase Antibodies (GAD65): Found in 70-90% of patients.
- Insulinoma-Associated Protein 2 Antibodies (IA-2): Detected in 50-70% of cases.
- Zinc Transporter 8 Antibodies (ZnT8): Specificity yet to be determined.
- Islet Cell Antibodies (ICA): Found in 44-100% of patients.
Causes of Type 2 Diabetes Mellitus
- Type 2 Diabetes Mellitus (DM) is strongly linked to genetic factors, with a high concordance rate of 70% to 90% among identical twins.
- The risk of developing Type 2 DM increases significantly if one or both parents have the condition.
- This type of diabetes is characterized by insulin resistance and inadequate insulin production, leading to elevated fasting plasma insulin levels.
Maturity Onset Diabetes of Young (MODY)
- MODY is a form of diabetes that is inherited in an autosomal dominant manner and typically presents with high blood sugar levels at an early age, usually before 25 years old.
- There are six recognized types of MODY: 1, 2, 3, 4, 5, and 6, with Type 3 being the most prevalent.
Genetic Defects of Pancreatic Cell Function in MODY
- MODY 1: Caused by a mutation in the HNF-4α gene; this type is rare.
- MODY 2: Involves a defect in the glucokinase gene; this type is less rare compared to MODY 1.
- MODY 3: Resulting from a mutation in the HNF-1α gene, which is located on chromosome 12; this type accounts for about two-thirds of all MODY cases.
- MODY 4: Caused by mutations in the PDX1 gene.
- MODY 5: Involves mutations in the HNF-1β gene.
- MODY 6: Caused by mutations in the NeuroD1 gene.
Type A Insulin Resistance Syndrome:
- This syndrome can be either autosomal dominant or recessive .
- It is caused by mutations in the INSR gene, which leads to a defective insulin receptor.
- Type 2 diabetes mellitus is characterized by insulin resistance resulting from various genetic factors and receptor problems, often associated with central obesity.
Key Clinical Features of Type A Insulin Resistance Syndrome:
- More common in females, especially those with primary amenorrhea or oligomenorrhea.
- Often linked with conditions such as Polycystic Ovary Syndrome (PCOS) or other endocrine disorders.
- Presence of ovarian cysts.
- Development of hirsutism.
- Appearance of acanthosis nigricans.
- Obesity is typically not present.
Clinical Features of DM
Classic Symptoms of DM include:
- Polyuria: More than 3 liters per day.
- Polydipsia: Increased thirst.
- Polyphagia: Increased hunger.
- Weight Loss and Asthenia: Weight gain typically indicates Type 2 diabetes mellitus.
- Recurring Infections: Such as urinary tract infections (UTIs) and vulvovaginitis.
- Postprandial Blurring of Vision: Blurred vision after meals.
Features of Maturity-Onset Diabetes of the Young (MODY) include:
- No Antibodies: Autoimmune markers are absent.
- No Obesity: Patients are not overweight.
- Rare Diabetic Ketoacidosis (DKA): DKA is not common in these cases.
- No Insulin Resistance: Insulin sensitivity is not impaired.
Investigations
1. HbA1c > 6.5%
- Normal HbA1c: Less than 5.6%.
- Impaired Glucose Tolerance: HbA1c of 5.7-6.4%.
- Reflection of Sugar Control: HbA1c indicates glucose control over the past 8-12 weeks.
- Retrospective Test: HbA1c is a retrospective measure.
- Long-Term Control: HbA1c shows long-term diabetes management.
- Patient Compliance: HbA1c is the best test for assessing patient adherence to treatment.
- Severity Measurement: HbA1c is the best indicator of diabetes severity.
- Preferred Diagnostic Test: HbA1c is the preferred test for diagnosing diabetes mellitus.
2. Blood Sugar Levels: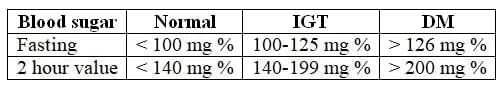
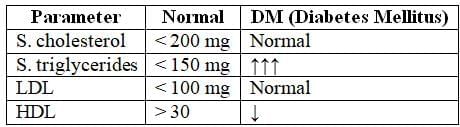
3. Lipid Profile:
Urinalysis for ketones, proteins, or sugar: The presence of microalbuminuria suggests kidney damage, and an ACE inhibitor should be started to delay progression to end-stage renal disease (ESRD).
- Serum Fructosamine: The best test for short-term control of diabetes, indicating glucose levels over the past 2 weeks.
- Diagnostic Criteria for Diabetes Mellitus:
- Symptoms of Diabetes: Such as increased thirst, frequent urination, and unexplained weight loss, along with a random blood glucose concentration >11.1 mmol/L (200 mg/dL).
- Fasting Plasma Glucose: >126 mg/dL on two separate occasions.
- HbA1c: >6.5% on two separate occasions.
- Oral Glucose Tolerance Test: Two-hour plasma glucose >200 mg/dL during an oral glucose tolerance test.
Treatment of Type 2 Diabetes Mellitus
- Exercise offers numerous benefits, such as reducing cardiovascular risk, lowering blood pressure, preserving muscle mass, decreasing body fat, and promoting weight loss.
- For individuals with type 1 or type 2 diabetes mellitus (DM), exercise also helps reduce plasma glucose levels during and after activity while improving insulin sensitivity.
- The American Diabetes Association (ADA) recommends 150 minutes per week of moderate aerobic exercise, spread over at least three days, combined with resistance training for patients with DM.
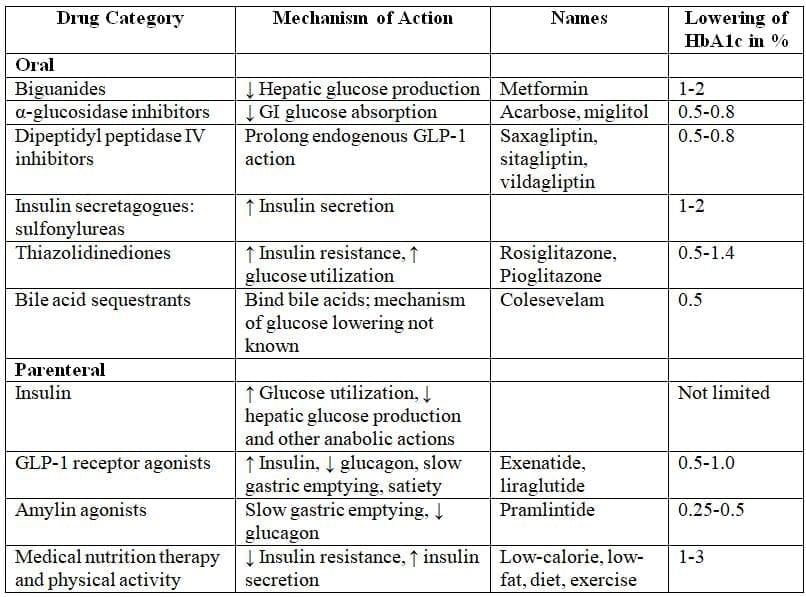
Oral Hypoglycemic Drugs
Algorithm for the Treatment of Type 2 Diabetes
Based on the Recommendations of the Consensus Panel of the American Diabetes AssociationEffect on weight:
- Metformin and DPP-4 inhibitors have a neutral effect on weight.
- GLP-1 receptor agonists and SGLT2 inhibitors can help with weight loss.
- Sulfonylureas, insulins, and pioglitazone are linked to weight gain.
Major side effects:
- Metformin can lead to lactic acidosis.
- Pioglitazone may cause fluid retention, increase fracture risk, and possibly lead to bladder cancer.
- GLP-1 receptor agonists can result in nausea and vomiting, and may be linked to pancreatitis.
- DPP-4 inhibitors might also carry a risk of pancreatitis.
- Bromocriptine is a recently approved antidiabetic medication.
- It enhances glycaemic control and glucose tolerance in obese type 2 diabetic patients.
- It helps reduce both fasting and postprandial plasma glucose levels.
Insulin Therapy
- Current insulin preparations are made using recombinant DNA technology and consist of the amino acid sequence of human insulin or its variations.
- In the United States, most insulin is available as U-100 (100 units/mL).
- Regular insulin is also available as U-500 (500 units/mL. for patients with severe insulin resistance.
- Insulin can be classified as either short-acting or long-acting.
Uses of insulin
- Type 2 diabetes that does not respond to oral hypoglycaemic drugs.
- Type 1 diabetes.
- Diabetic ketoacidosis.
- Non-ketotic hyperosmolar coma.
Insulin Delivery
- The insulin pump is regarded as the most effective delivery method.
- Insulin pen.
- Inhaled insulin.
- Multi-dose vial.
Sites for insulin administration
- Abdominal wall
- Thigh and arm
- Buttocks
- The abdomen is preferred as it absorbs insulin faster than other areas.
Preparation of Insulin

Side Effect of Insulin Usage
- Somogyi phenomenon: It refers to a condition where there is a low blood sugar level in the early morning, followed by a rebound high blood sugar level before breakfast.
- Dawn phenomenon: This condition is characterized by high blood sugar levels in the early morning before breakfast.
New Trends in Management of Diabetes Mellitus:
- Whole pancreas transplantation, often performed with a kidney transplant, can restore normal glucose levels.
- If pancreas transplantation is not feasible, beta cell extract can be infused into the recipient's portal vein.
- After a successful pancreas transplant, patients may experience improved blood sugar control and a reduced need for insulin or other diabetes medications.
Complications of Diabetes Mellitus that may be reversed/healed:
- Early diabetic nephropathy: This condition, characterized by the presence of microalbuminuria, can be reversed with proper management.
- Diabetic neuropathy: Nerve damage caused by diabetes can heal with appropriate treatment and lifestyle changes.
Complications that cannot be reversed/healed:
- Diabetic retinopathy: Damage to the retina due to diabetes is irreversible and can lead to vision loss.
- Peripheral vascular disease: Poor circulation in the limbs caused by diabetes cannot be reversed, leading to various complications.
- Closed-loop pumps are advanced devices that automatically adjust insulin delivery based on real-time blood sugar levels, improving glycemic control.
- Bariatric surgery is a surgical option for severely obese individuals with type 2 diabetes. According to the American Diabetes Association (ADA) guidelines, it is recommended for patients with a body mass index (BMI) over 35 kg/m² who have diabetes.
Acute Complications of Diabetes Mellitus
- Complications of Diabetic Ketoacidosis: Cerebral edema. This is the most severe complication of diabetic ketoacidosis, especially in children. Other complications include venous thrombosis, acute respiratory distress syndrome (ARDS), myocardial infarction (MI), and acute gastric dilatation.
Treatment for Diabetic Ketoacidosis includes:
- Fluids: Administering 0.9% saline to rehydrate the patient.
- Insulin: Regular insulin is given intravenously in cases of diabetic ketoacidosis.
- Treating underlying causes: Identifying and treating causes such as noncompliance or infections with antibiotics.
- Potassium replacement: Monitoring and replacing potassium levels, as they may drop during treatment.
- Sodium bicarbonate: Administering sodium bicarbonate if the pH is less than 7.2 despite adequate treatment.
Non-ketotic Hyperosmolar Coma
Typical Patient Presentation:
- Demographics: Usually elderly
- History:
- Polyuria lasting several weeks
- Weight loss
- Reduced oral intake
- Mental confusion
Clinical Signs
- Rapid heart rate (tachycardia)
- Low blood pressure (hypotension)
- Dehydration
- Altered mental status or coma
Diagnostic Criteria
- Blood glucose >600 mg/dL
- Serum osmolality >310 mOsm/kg
- No acidosis present
- Bicarbonate >15 mEq/L
- Normal anion gap
Management
- Fluid Therapy:
- Correct total fluid deficit (9–10 L) over 1–2 days
- Start with normal saline to stabilize hemodynamics (target systolic BP >90 mm Hg)
- Switch to 0.45% saline after stabilization
- Insulin Therapy: Administer regular insulin intravenously
- Thromboprophylaxis: Use subcutaneous heparin to prevent venous thrombosis due to high risk
Laboratory Diagnosis of Coma in Diabetic Patients
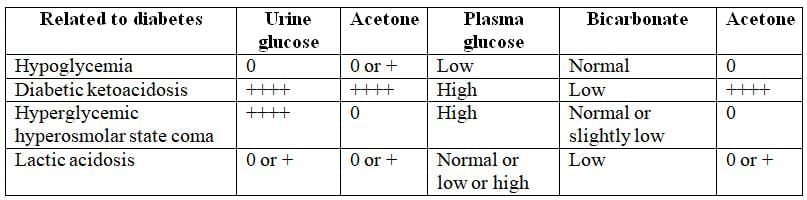
Lactic Acidosis
Lactic acidosis is a condition marked by low pH in blood and tissues, accompanied by elevated lactate levels, often signaling tissue hypoxia, hypoperfusion, or acute circulatory failure. It is frequently observed in type 2 diabetes patients treated with metformin.
Key Features
- Blood pH below 7.3
- Serum lactate exceeding 5 mmol/L
- Serum bicarbonate below 15 mEq/L
- High anion gap
- Absence of serum ketones
- Symptoms include rapid, deep breathing (hyperventilation), vomiting, and abdominal pain
Treatment Approaches
- Address underlying causes such as shock, fluid volume deficits, impaired cardiac function, or sepsis. Identify and treat the sepsis source or resect ischemic tissues if needed.
- Administer intravenous sodium bicarbonate if pH remains below 7.2 despite adequate fluid resuscitation.
- Consider thiamine supplementation, as thiamine deficiency may contribute to cardiovascular issues and lactic acidosis. Thiamine repletion can lead to significant, potentially life-saving improvement.
Wolfram Syndrome/ DIDMOAD
- Wolfram Syndrome, also known as DIDMOAD syndrome, is caused by a mutation in the WFS1 gene.
The symptoms of Wolfram Syndrome include:
- Central diabetes insipidus: A condition where the body cannot properly regulate water balance, leading to excessive urination and thirst. Diabetes mellitus: A form of diabetes characterized by high blood sugar levels due to insulin deficiency or resistance.
- Optic atrophy: Degeneration of the optic nerve, leading to vision problems.
- Deafness: Hearing loss due to the degeneration of auditory pathways or structures.
Long-Term Complications of Diabetes
A person with diabetes is 25 times more likely to experience blindness.
On Fundus Examination
- The initial sign of diabetic retinopathy is the presence of microaneurysms in the inner layers of the retina.
- Non-proliferative retinopathy can progress to macular swelling and gradual vision loss.
- Proliferative diabetic retinopathy is marked by the formation of new blood vessels due to low oxygen levels in the retina, often near the optic nerve or macula. These fragile vessels are prone to rupture, leading to bleeding, scarring, and retinal detachment.
Not all individuals with non-proliferative retinopathy will develop the proliferative form, but those with severe non-proliferative retinopathy face a higher risk within five years.
This underscores the critical need for early detection and intervention in diabetic retinopathy.
Elevated levels of homocysteine and creatinine may indicate an increased risk for developing diabetic retinopathy.
Treatment options for neovascularization include:
- Pan-retinal photocoagulation using ND-YAG laser.
- Injection of anti-VEGF agents such as Bevacizumab.
The most effective strategy for reducing both microvascular and macrovascular complications in type 2 diabetes mellitus is through managing blood pressure, rather than focusing solely on blood sugar levels.
Infections
- Rhinocerebral mucormycosis
- Invasive aspergilloma
- Malignant otitis externa
- Emphysematous pyelonephritis
Diabetic Neuropathy
The first area affected in diabetic neuropathy is the sensory system, leading to a loss of sensation, with vibration sense being the first to deteriorate. This is typically assessed using a 128 Hz tuning fork.
Conditions that may result in a stamping gait include:
- Diabetic neuropathy
- INH (isoniazid) toxicity
- Vitamin B6 (B6) toxicity
- Tabes dorsalis
Motor Symptoms
The first cranial nerve to be affected in diabetic neuropathy is the third cranial nerve, leading to:
- Ptosis (drooping of the eyelid)
- Diplopia (double vision)
- Squinting, although the pupils may still react normally to light.
Autonomic Symptoms
- Silent myocardial infarction, which can lead to sudden death.
- Postural hypotension
- Resting tachycardia
- Nocturnal diarrhoea
- Constipation
- Gastroparesis
Postural hypotension is characterized by a significant drop in blood pressure when transitioning from a lying down to a standing position within three minutes:
- Systolic blood pressure drop > 20 mm Hg
- Diastolic blood pressure drop > 10 mm Hg
Patients may experience symptoms such as dizziness, vertigo, and faintness.
Treatment options for postural hypotension include:
- Wearing elastic stockings
- Increasing dietary salt intake
- Medications such as midodrine (oral)
Note: Clinical Alerts:
- The most consistent indicator of hypoglycemia in diabetics is sweating.
- Non-selective beta-blockers should be avoided in diabetic patients due to potential adverse effects.
Diabetic Nephropathy
The American Diabetes Association has shifted away from using the terms microalbuminuria and macroalbuminuria, opting instead for the term albuminuria. This is defined as a spot urinary albumin to creatinine ratio exceeding 30 mg/g Cr.
Albuminuria is a significant risk factor for:
- Cardiovascular disease
- Progression of chronic kidney disease (CKD)
In the initial five years of diabetes mellitus, thickening of the glomerular basement membrane is observed. After 5-10 years of type 1 diabetes mellitus:
- 40% of individuals begin to excrete small amounts of albumin in their urine.
Microalbuminuria is characterized by:
- 30-299 mg/day in a 24-hour urine collection
- 30-299 µg/mg creatinine in a spot urine collection (this method is preferred).
Microalbuminuria is a critical risk factor for the development of macroalbuminuria (greater than 300 mg/day) and cardiovascular disease.
Once macroalbuminuria is diagnosed, there is a consistent decline in glomerular filtration rate (GFR), with 50% of individuals progressing to end-stage renal disease (ESRD) within 7-10 years.
The most prevalent histopathological finding in diabetic nephropathy is diffuse glomerulosclerosis, with the hallmark feature being nodular glomerulosclerosis.
Stages of chronic kidney disease (CKD) based on GFR are as follows:
- Stage I: GFR ≥ 90 mL/min/1.73 m²
- Stage II: GFR 60-89 mL/min/1.73 m²
- Stage III: GFR 30-59 mL/min/1.73 m²
- Stage IV: GFR 15-29 mL/min/1.73 m²
- Stage V: GFR < 15 ml/min/1.73 m²
Treatment with ACE inhibitors has been demonstrated to slow the progression of diabetic nephropathy.
Diabetic Dermatopathy
The most prevalent skin conditions observed in individuals with diabetes include:
- Xerosis (dry skin) accompanied by pruritus (itching).
- Prolonged wound healing.
- Pigmented pretibial papules that initially manifest as red spots and subsequently evolve into circular dark patches.
- Necrobiosis lipoidica diabeticorum, characterized by lesions with superficial ulcers or sores on the shins of young women.
- Acanthosis nigricans is characterized by dark, velvety patches that typically appear in areas such as the neck, armpits, and the back of the arms and legs.
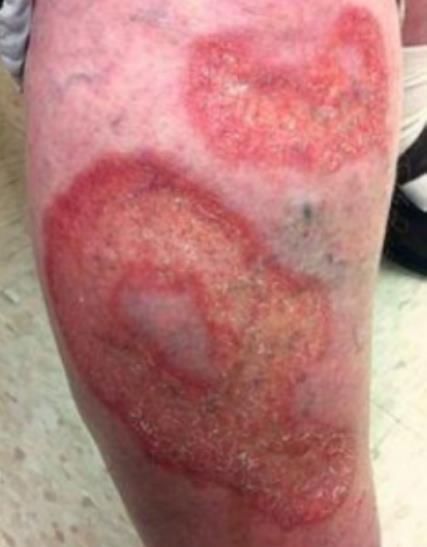 Necrobiosis lipoidica diabeticorum
Necrobiosis lipoidica diabeticorum
Summary of Complications of Diabetes Mellitus
Microvascular Complications:
Retinopathy
- Non-proliferative retinopathy
- Proliferative retinopathy
Ischemic cardiomyopathy
Macrovascular Complications:
- Coronary heart disease (occurs 3-5 times more frequently than in the general population)
- Peripheral arterial disease (occurs 100 times more frequently than in the general population)
- Cerebrovascular disease
Treatment Goals for Adults with Diabetes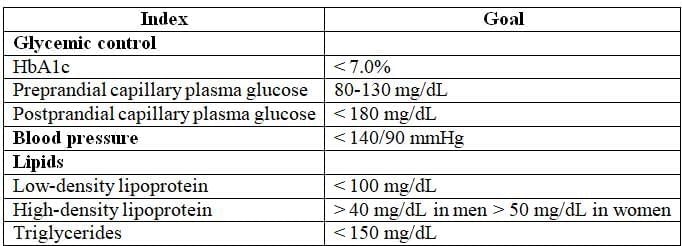
Key Landmark Trials in Diabetes Mellitus
The DCCT demonstrated that better glycemic control resulted in significant reductions in various complications:
- 47% reduction in both non-proliferative and proliferative retinopathy
- 39% reduction in microalbuminuria
- 54% reduction in clinical nephropathy
- 60% reduction in neuropathy
These improvements also delayed the onset of early diabetic complications, with a slight trend towards fewer macrovascular events, although not statistically significant.
The UKPDS found that each percentage point decrease in HbA1c was associated with a 35% reduction in microvascular complications. A key finding was that strict blood pressure control significantly reduced both macrovascular and microvascular complications, often more so than blood sugar control. Achieving moderate blood pressure targets (114/82 mmHg) lowered the risks of diabetes-related death, stroke, microvascular complications, retinopathy, and heart failure, with risk reductions between 32% and 56%.
Metabolic Syndrome
Metabolic syndrome is also known as syndrome X.
Centripedal Obesity
- Waist circumference greater than 102 cm in males.
- Waist circumference greater than 88 cm in females.
Hyperlipidemia
- Characterised by elevated triglycerides.
- Defined by low HDL (high-density lipoprotein) levels.
Hypertension
- Systolic blood pressure of 130 mm Hg or higher.
- Diastolic blood pressure of 80 mm Hg or higher.
Fasting Plasma Glucose
- Fasting plasma glucose level of 100 mg/dL or higher.
- Previously diagnosed with type 2 diabetes.
Distinction from Coronary Syndrome X:
- Metabolic syndrome should not be confused with coronary syndrome X, which is related to microvascular dysfunction.
- In coronary syndrome X, there is no obstruction of major blood vessels.
- Percutaneous coronary intervention is not effective for treating coronary syndrome X.
- The preferred treatment for coronary syndrome X is nitrates.
Environmental Factors Influencing Insulin Resistance:
- Obesity is the most prevalent environmental factor contributing to insulin resistance.
Overview of Syndrome Z:
- Syndrome Z is a condition that combines aspects of metabolic syndrome with obstructive sleep apnea.
Adrenal Gland
- The right adrenal gland is typically triangular or pyramidal in shape, whereas the left adrenal gland has a semilunar shape.
- Each adrenal gland weighs approximately 4-5 grams.
- Adrenal glands consist of two main parts: the outer adrenal cortex and the inner medulla, both of which are responsible for hormone production.
- The adrenal cortex primarily produces hormones such as cortisol, aldosterone, and androgens.
- The adrenal medulla mainly secretes adrenaline and noradrenaline.
- The medulla is directly regulated by nerve signals, while the cortex is controlled by neuroendocrine hormones from the pituitary gland. The activity of the pituitary gland, in turn, is influenced by the hypothalamus and the renin-angiotensin system.
Hormonal Functions and Associated Disorders
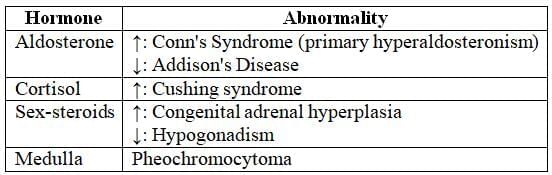
Congenital Adrenal Hyperplasia
- Inheritance Pattern: Autosomal recessive
- Enzyme Deficiency: 21-a-hydroxylase
- Cholesterol serves as the substrate for the synthesis of adrenal hormones.
- Both aldosterone and cortisol require the enzyme 21-a-hydroxylase for their production.
- In cases of 21-a-hydroxylase deficiency, the levels of aldosterone and cortisol decrease. This deficiency leads to the diversion of cholesterol towards the production of sex steroids, resulting in virilization of female infants.
- The decrease in these hormones triggers a negative feedback loop, causing an increase in ACTH production. This elevated ACTH explains the hyperpigmentation observed around the genital area of girls, as ACTH has effects similar to MSH (melanocyte-stimulating hormone).
Clinical Features of Congenital Adrenal Hyperplasia (CAH)
- Salt Wasting
- Hypoglycemia
- Virilization of Female Infants: This leads to ambiguous genitalia at birth.
- Male Infants typically have normal genitalia at birth. However, they may later develop precocious puberty, characterized by a short, muscular stature and phallic enlargement, often referred to as 'Hercules appearance'.
- Screening: Elevated levels of 17-keto steroids in the urine of the mother.
- Investigation of Choice (IOC): Elevated levels of 17-keto steroids in the baby, confirmed through cordocentesis.
- Treatment. Initiate dexamethasone in the mother, as it reduces the severity of the disease in the fetus.
Other Defects in CAH
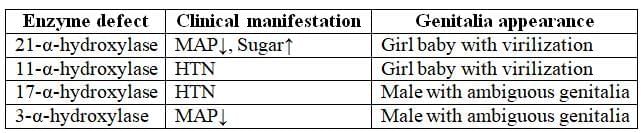
Addison's Disease
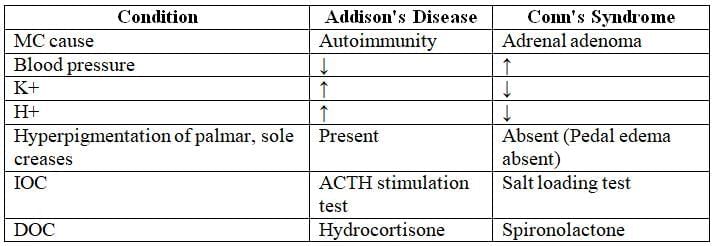
Addison’s disease is marked by reduced levels of aldosterone, cortisol, and sex steroids, with aldosterone deficiency symptoms appearing earliest.
Causes of Addison’s Disease
- Most common infectious causes: HIV, followed by tuberculosis (TB) (Ref: Harrison’s 20th ed., p. 309).
- Autoimmune etiology is the leading cause globally.
- Addisonian crisis, triggered by abrupt cessation of steroid therapy.
- Waterhouse-Friderichsen syndrome.
- Secondary Addison’s disease due to Sheehan’s syndrome (pituitary infarction from obstetric causes).
- Non-obstetric pituitary damage, known as Simmonds’ disease.
Clinical Manifestations
- Salt wasting from sodium and water loss.
- Polyuria (>3 L/day).
- Decreased mean blood pressure, causing dizziness, presyncope, or syncope.
- Hyperkalemia.
- Metabolic acidosis.
- Elevated ACTH, mimicking melanocyte-stimulating hormone, leading to hyperpigmentation of the oral mucosa, palmar/solar creases, and old surgical scars.
- Hypoglycemia.
- Loss of pubic and axillary hair.
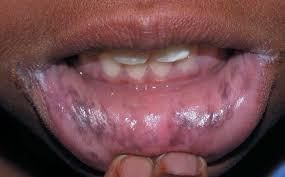 Hyperpigmentation of oral mucosa in Addison's disease
Hyperpigmentation of oral mucosa in Addison's disease
Diagnostic Investigations
- Serum sodium decreased (normal: 135–145 mEq/L); potassium increased (normal: 3.5–5.5 mEq/L).
- Gold standard test: ACTH stimulation test (Cosyntropin test), showing failure of adrenal gland response.
- Abdominal CT to detect adrenal gland damage.
- GeneXpert for Mycobacterium tuberculosis (MTB).
Treatment
- Fludrocortisone: Enhances sodium and water retention.
- Dexamethasone: Mimics cortisol to alleviate hypoglycemic symptoms.
- Drug of choice for Addison’s disease: Hydrocortisone (15–30 mg daily, in 2–3 divided doses).
- Drug of choice for Addisonian crisis: Intravenous hydrocortisone with fluid resuscitation.
- Note: Dextrose administration may cause glucose fever.
Conn's Syndrome
- Pituitary adenoma:. tumor in the pituitary gland causing increased production of cells and their products, leading to higher levels of ACTH and cortisol, known as Cushing disease (endogenous cause).
Clinical Features of Conn's Syndrome
Hypertension
Hypokalemia (low K+): Muscle cramps, fatigue
Reduced H+ levels: Metabolic alkalosis
No pedal edema
Polyuria and polydipsia
Investigations
Increased serum sodium (Na+)
Decreased serum potassium (K+)
Abdominal CT: Tumor in zona glomerulosa
Screening test: Plasma aldosterone-to-renin ratio (hypertension suppresses renin via negative feedback)
Gold standard test: Saline infusion test or salt loading test
Treatment
- Definitive treatment: Surgical resection of adrenal tumor
- Drug of choice: Spironolactone
Comparison of Primary Addison's and Conn's Syndrome
Conditions for Isolated Diastolic Hypertension
- Essential hypertension.
- Myxedema.
- Signs and Symptoms
Cushing Syndrome
Cushing Syndrome is marked by the absence of normal daily cortisol fluctuations and persistently high cortisol levels. Its causes include:
- Iatrogenic Steroids: The most frequent cause, where external steroid use suppresses ACTH levels, leading to elevated cortisol (exogenous cause).
- Ectopic ACTH Production: Often due to bronchial carcinoid tumors, this results in continuous adrenal gland stimulation, causing excessive cortisol production.
- Pituitary Adenoma (Cushing Disease): A tumor in the pituitary gland increases ACTH and cortisol production, classified as an endogenous cause.
- Adrenal Adenoma: A tumor in the adrenal gland boosts cortisol production directly, but ACTH levels remain low due to the tumor's location in the adrenal gland.
Clinical Features of Cushing Syndrome
- Earliest finding is loss of diurnal variation of cortisol production.
Body fat:
- Weight gain, central obesity, rounded face, fat pad on back of neck (often called "buffalo hump").
Skin:
- Facial plethora, thin and brittle skin, easy bruising, broad and purple stretch marks, acne, hirsutism.
Bone:
- Osteopenia, osteoporosis (vertebral fractures), decreased linear growth in children.
Muscle:
- Weakness, proximal myopathy (notable atrophy of gluteal and upper leg muscles).
Cardiovascular System:
- Diastolic hypertension, hypokalaemia, oedema, atherosclerosis.
Metabolism:
- Glucose intolerance/diabetes, dyslipidaemia.
Reproductive system:
- Decreased libido; in women, amenorrhea (due to cortisol inhibiting gonadotropin release).
Central nervous system:
- Irritability, emotional lability, depression, sometimes cognitive defects; in severe cases, paranoid psychosis.
Blood and Immune system:
- Increased susceptibility to infections, increased white blood cell count, eosinopenia, hypercoagulation with increased risk of deep vein thrombosis and pulmonary embolism.
Cushing's Syndrome Investigation
- Screening Test: Measure 24-hour urinary cortisol levels (elevated up to twice the normal range) or salivary cortisol levels.
- Investigation of Choice: Low-dose dexamethasone suppression test (plasma cortisol >50 nmol/L after 0.5 mg dexamethasone every 6 hours for 2 days).
- Imaging:
- MRI of the head to assess for pituitary adenoma.
- CT of the chest to exclude lung cancer.
- CT of the abdomen to evaluate for adrenal adenoma.
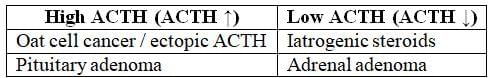
- ACTH Levels:
- Normal or high (>15 pg/mL): Indicates ACTH-dependent Cushing's syndrome.
- Low (<5 pg/mL): Indicates ACTH-independent Cushing's syndrome.
Lab Investigations in Common Etiologies of Cushing's Syndrome
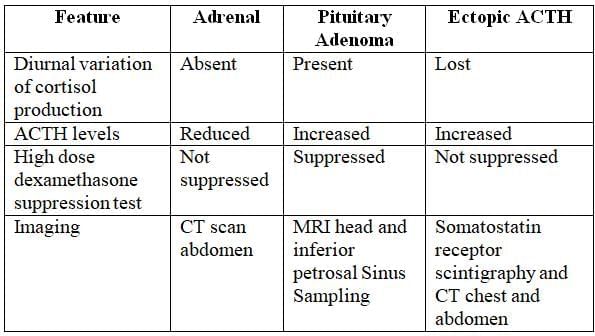
Polyglandular Autoimmune Syndrome:
Type 1: Autoimmune Polyendocrinopathy Candidiasis (ectodermal dystrophy).
- Autosomal recessive with a defect in T-cell function, presenting in childhood.
- Mucocutaneous candidiasis.
- Hypoparathyroidism with dystrophy of nails and teeth.
Type 2:
- Addison's disease is an autoimmune condition that leads to adrenal insufficiency.
- It is often associated with other autoimmune disorders such as Type 1 diabetes mellitus, autoimmune thyroid disease, and vitiligo.
- The combination of Addison's disease and hypothyroidism is known as Schmidt's syndrome.
- Other conditions that may occur alongside Addison's disease include alopecia areata and Sjogren's syndrome.
Conditions with Hyperpigmentation (ACTH ↑):
- Ectopic ACTH secretion, such as in oat cell cancer.
- Pituitary adenoma leading to Cushing's syndrome.
- Nelson syndrome.
Conditions Characterized by No Hyperpigmentation (ACTH ↓):
- Addisonian crisis
- Pituitary damage resulting in secondary Addison's disease, characterized by alabaster-colored pale skin.
- Tertiary Addison's disease.
|
13 docs|47 tests
|
FAQs on Endocrinology- 1 Chapter Notes - Medicine - NEET PG
| 1. What is acromegaly, and what are its main causes? |  |
| 2. How does diabetes mellitus affect long-term health, and what are some common complications? |  |
| 3. What are the primary treatment goals for adults with diabetes? |  |
| 4. What is the significance of acanthosis nigricans in diabetes? |  |
| 5. What key landmark trials have influenced the management of diabetes mellitus? |  |














