Sure Shot Questions for Board Exams: Metals & Non-metals - Class 10 PDF Download
| Table of contents |

|
| Introduction |

|
| Key Questions |

|
| Important Topics for Preparation |

|
| Study Tips |

|
Introduction
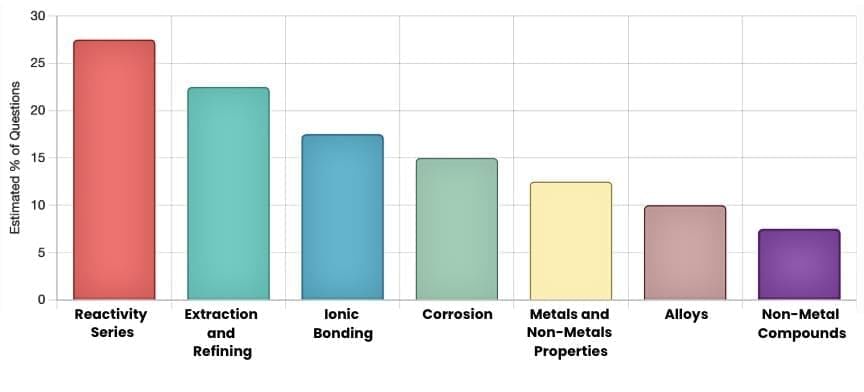
The CBSE Class 10 chapter on Metals and Non-Metals is a critical part of the Chemistry syllabus, covering key concepts like reactivity, extraction, corrosion, and properties of metals and non-metals. By analyzing previous year question papers (2015–2025), we’ve identified recurring patterns and high-weightage topics, such as reactivity series, thermite reactions, and rusting, which dominate exam questions. These trends, combined with their syllabus importance, guide our predictions for questions likely to reappear. The following chart and insights highlight the most probable topics, supported by examples from past papers, to streamline your preparation for the upcoming exam.
Here's a chart showing the frequency of key topics in CBSE Class 10 "Metals and Non-Metals" based on previous year paper analysis:
Key Questions
Q1: A metal, M, displaces iron from aqueous solution of ferrous sulphate but fails to do so in case of aqueous solution of aluminium sulphate. The metal M is:
(a) Magnesium
(b) Copper
(c) Lead
(d) Zinc
 View Answer
View Answer 
Ans: (d) Zinc
- Reactivity series: K > Na > Ca > Mg > Al > Zn > Fe > Pb > Cu.
- Metal M displaces Fe from FeSO₄, so M is above Fe (Mg, Al, Zn).
- M does not displace Al from Al₂(SO₄)₃, so M is below Al.
- Zinc (Zn) fits: Zn + FeSO₄ → ZnSO₄ + Fe; no reaction with Al₂(SO₄)₃.
Q2: Study the following cases:
(i) CuSO₄ + Mg →
(ii) FeSO₄ + Pb →
(iii) CaSO₄ + Al →
(iv) ZnSO₄ + Ca →
The case/cases in which new product(s) will form is/are:
(a) Only (i)
(b) Only (iii)
(c) (i) and (iv)
(d) (i), (ii) and (iv)
 View Answer
View Answer 
Ans: (a) Only (i)
- Reactivity series: Mg > Al > Zn > Fe > Pb > Cu.
- (i) Mg displaces Cu: Mg + CuSO₄ → MgSO₄ + Cu.
- (ii) Pb is below Fe, no reaction.
- (iii) Al is below Ca, no reaction.
- (iv) Ca is below Zn, no reaction.
Q3: Assertion - Reason based questions: These questions consist of two statements — Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below: (CBSE 2024)
Assertion ( A): A piece of zinc metal gets reddish-brown coating when kept in copper sulphate solution for some time.
Reason (R): Copper is more reactive metal than zinc.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
 View Answer
View Answer 
Ans: (c)
Assertion (A): A piece of zinc metal gets a reddish-brown coating when kept in copper sulfate solution for some time. This is true. When zinc is placed in a copper sulfate solution, a displacement reaction occurs where zinc displaces copper from copper sulfate. The copper then deposits on the zinc surface as a reddish-brown coating.
Reason (R): Copper is more reactive than zinc. This is false. In the reactivity series, zinc is more reactive than copper, which is why zinc can displace copper from copper sulfate.
Since Assertion (A) is true but Reason (R) is false, the correct answer is (c) (A) is true, but (R) is false
Q4: What would a student report nearly after 30 minutes of placing duly cleaned strips of aluminium, copper, iron and zinc in freshly prepared iron sulphate solution taken in four beakers? (2019)
 View Answer
View Answer 
Ans: After approximately 30 minutes, the student would observe that the strip of iron would start to show signs of corrosion or rusting. This is because iron is more reactive than copper, aluminium, and zinc and will displace the iron from the iron sulphate solution, forming iron oxide (rust) on its surface.
Explanation: When cleaned metal strips of aluminium (Al), copper (Cu), iron (Fe), and zinc (Zn) are each placed into separate beakers containing freshly prepared iron(II) sulphate (FeSO₄) solution, here’s what the student would observe after about 30 minutes:
Aluminium strip (Al)
- Above Fe in the reactivity series:
Al+FeSO4⟶Al2(SO4)3+Fe - Expected change: A brownish deposit of iron on the aluminium strip and possible fading of the greenish FeSO₄ solution.
- Above Fe in the reactivity series:
Copper strip (Cu)
- Below Fe in the reactivity series:
- Copper cannot displace iron from its salt.
- Expected change: No reaction; the copper strip remains unchanged, and the solution color stays the same.
- Below Fe in the reactivity series:
Iron strip (Fe)
- Same metal as in the solution:
- No net displacement reaction occurs because the metal and the dissolved ion are both iron.
- Expected change: No reaction; no visible change in the metal strip or the solution.
- Same metal as in the solution:
Zinc strip (Zn)
- Above Fe in the reactivity series:Zn+FeSO4⟶ZnSO4+ Fe
- Expected change: A brownish deposit of iron on the zinc strip and the FeSO₄ solution may lose its greenish color as Fe²⁺ ions are displaced.
- Al in FeSO₄: Brownish deposit (iron) forms on the strip.
- Cu in FeSO₄: No change.
- Fe in FeSO₄: No change.
- Zn in FeSO₄: Brownish deposit (iron) forms on the strip.
Q5: A pale green solution of ferrous sulphate was taken in four separate test tubes marked I, II, III and IV. Pieces of Cu, Zn and Al were dropped in test tubes II, III and IV respectively. In which case(s)
(a) Does the colour of the ferrous sulphate solution match with the colour in test tube (I)? Give reason.
(b) the colour of the ferrous sulphate solution will fade and a black mass will be deposited on the surface of the metal. (2019 C)
 View Answer
View Answer 
Ans: (a) The colour of the ferrous sulphate solution in test tube (I) will match the colour of the solution in test tube II when a piece of copper is dropped in it. This is because copper is less reactive than iron and will not displace iron from ferrous sulphate solution, resulting in no change in the colour of the solution.
No reaction: Cu + FeSO4 → CuSO4 + Fe
(b) The colour of the ferrous sulphate solution will fade and a black mass will form on the metal surface in the following cases:
Zinc displacing iron:
Zn+FeSO4 ⟶ ZnSO4+Fe (s)- The FeSO₄ solution fades because Fe²⁺ ions are replaced by Zn²⁺.
- The displaced iron is deposited on the zinc strip, often appearing black or brown in color.
Aluminium displacing iron:
2Al+3FeSO4⟶Al2(SO4)3+3Fe (s)- Similarly, the pale green color of FeSO₄ solution fades as iron ions are displaced.
- The black/brown deposit of iron forms on the aluminium strip.
Q6: Cinnabar is an ore of a metal 'X'. When this ore is heated in air, it is first converted into oxide of 'X' (XO) and then reduced to metal 'X' on further heating. Identify metal 'X' and write chemical equations for the reactions that occur in the above processes.
 View Answer
View Answer 
Ans:
Metal X: Mercury (Hg).
Equations:
- 2HgS + 3O₂ → 2HgO + 2SO₂
- 2HgO → 2Hg + O₂
Explanation:
- Cinnabar (HgS) is roasted to form mercury(II) oxide (HgO).
- HgO decomposes on heating to yield mercury metal.
Q7: Name the ore of mercury and state the form in which it is found in nature. Write the chemical equations along with the condition required for the reactions involved in the extraction of mercury from its ore. (2024)
 View Answer
View Answer 
Ans: Ore of mercury: Cinnabar
Form in nature: Sulphide ore
The extraction of mercury from cinnabar involves the following chemical reactions:
- When heated in air, cinnabar (HgS) converts to mercuric oxide (HgO):
- 2HgS(s) 3O2(g) → 2HgO(s) 2SO2(g)
- Further heating of mercuric oxide produces mercury:
- 2HgO(s) → 2Hg(l) O2(g)
Q8: A metal ‘X’ is used in the thermit process. When ‘X’ is heated with oxygen, it gives an oxide ‘Y’, which is amphoteric in nature. ‘X’ and ‘Y’ respectively are: (2024)
(a) Mn, MnO2
(b) Al, Al2O3
(c) Fe, Fe2O3
(d) Mg, MgO
or
A metal ‘X’ is used in thermite process. When X is burnt in air it gives an amphoteric oxide 'Y'. 'X' and 'Y' are respectively:
(a) Fe and Fe2O3
(b) Al and Al2O3
(c) Fe and Fe3O4
(d) Al and Al3O4 (CBSE 2023)
 View Answer
View Answer 
Ans: (b)
Metal ‘X’ is aluminium, used in the thermit process. When aluminium is heated with oxygen, it produces aluminium oxide (Y), which is amphoteric. This means it can react with both acids and bases.
- Metal X: Aluminium (Al)
- Oxide Y: Aluminium oxide (Al2O3)
- Nature: Amphoteric
In the thermite process, aluminum (Al) is used because of its high reactivity and ability to reduce metal oxides, such as iron oxide, to produce molten iron.
When aluminum is burnt in air, it forms aluminum oxide (Al₂O₃), which is an amphoteric oxide (meaning it can react with both acids and bases).
Therefore, X is aluminum (Al) and Y is aluminum oxide (Al₂O₃), making the correct answer (b) Al and Al₂O₃.
Q9: The following questions are source-based/case-based questions. Read the case carefully and answer the questions that follow. Metals are required for a variety of purposes. For this, we need their extraction from their ores. Ores mined from the earth are usually contaminated with many impurities which must be removed before the extraction of metals. The extraction of pure metal involves the following steps:
(1) Concentration of ore
(2) Extraction of metal from the concentrated ore
(3) Refining of metal
(a) Name an ore of mercury and state the form in which mercury is present in it.
(b) What happens to zinc carbonate when it is heated strongly in a limited supply of air?
(c) The reaction of a metal A with Fe2O3 is highly exothermic and is used to join railway tracks.
(I) Identify the metal A and name the reaction taking place.
(II) Write the chemical equation or the reaction of metal A with Fe2O3. (2023)
 View Answer
View Answer 
Ans: (a) An ore of mercury is cinnabar, and mercury is present in it in the form of mercury sulfide (HgS).
(b) When zinc carbonate (ZnCO3) is heated strongly in a limited supply of air, it undergoes thermal decomposition to produce zinc oxide (ZnO), carbon dioxide (CO2), and water (H2O):
ZnCO3(s) → ZnO(s) + CO2(g)
(c) (I) The metal is aluminium (Al), and the reaction is called the thermite reaction.
(II) The chemical equation for the reaction of iron (A) with iron(III) oxide (Fe2O3) is:
2Al(s) + Fe2O3(s) → 2Fe(s) + Al2O3(s)
This reaction is highly exothermic and is used in various industrial applications, including joining railway tracks due to its high heat generation and the ability to melt and fuse metals.
Q10: (a) Show the formation of magnesium chloride by electron transfer. Write the name of the cation and anion present in the compound formed. (Atomic Number of Mg=12, Cl=17)
OR
(b) How is zinc extracted from its ore? Name the processes involved in the extraction and write chemical equations for the reactions that occur during these processes.
 View Answer
View Answer 
Ans: (a)
Formation:
- Mg (2,8,2) → Mg²⁺ (2,8) + 2e⁻
- Cl (2,8,7) + e⁻ → Cl⁻ (2,8,8) [×2 for 2Cl]
- Mg + 2Cl → MgCl₂ (Mg²⁺[Cl⁻]₂)
Cation: Mg²⁺; Anion: Cl⁻
Explanation:
- Mg donates 2 electrons to two Cl atoms, forming ionic MgCl₂.
Q11: Write the electron-dot structures of (i) sodium, and (ii) oxygen. Using these structures, show the formation of sodium oxide. Mark the anion and cation present in this compound. (At. No. - Sodium = 11, Oxygen = 8)
 View Answer
View Answer 
Ans:
Electron-dot structures:
- Sodium (Na): 2,8,1 → Na·
- Oxygen (O): 2,6 → :Ö:
Formation of Na₂O:
- 2Na· → 2Na⁺ + 2e⁻
- :Ö: + 2e⁻ → [Ö]²⁻
- 2Na⁺ + [Ö]²⁻ → Na₂O
Cation: Na⁺; Anion: O²⁻
Each Na donates 1 electron to O, forming ionic Na₂O.
Q12: (a) Write the electron dot structure of Ca (At. No. 20) and O (At. No. 8).
(b) Show the formation of calcium oxide by the transfer of electrons.
(c) Name the ions present in this compound.
(d) List four important characteristics of this compound. (2020)
 View Answer
View Answer 
Ans: 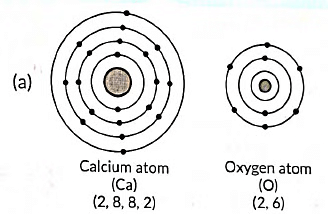
(b) The formation of calcium oxide (CaO) involves the transfer of electrons. Calcium (Ca) donates two electrons to oxygen (O) to form Ca2+ cation and O2- anion. The ionic bond is formed between these ions to create calcium oxide.
(c) In calcium oxide (CaO), the ions present are Ca2+ (calcium cation) and O2- (oxygen anion).
(d) Four important characteristics of calcium oxide (CaO) are:
- It is a white, crystalline solid.
- It has a high melting and boiling point.
- It is an ionic compound.
- It is commonly used as a desiccant and in cement production.
Q13: Describe an activity to find out the conditions under which iron rusts. (2017)
 View Answer
View Answer 
Ans: To find out the conditions under which iron rusts, you can perform the following activity:
- Take three test tubes and label them as A, B, and C.
- Fill test tube A with water, test tube B with water and oil, and test tube C with water and salt.
- Place a small piece of iron nail in each test tube and allow them to stand undisturbed for a few days.
- Observe the test tubes regularly and note any changes in the appearance of the iron nails.
- After a few days, check for the presence of rust on the iron nails in each test tube.
- Analyze the results and determine the conditions under which iron rusts.
Q14: A metal 'X' combines with a non-metal 'Y' by the transfer of electrons to form a compound Z.
(i) State the type of bond in compound Z.
(ii) What can you say about the melting point and boiling point of compound Z?
(iii) Will this compound dissolve in kerosene or petrol?
(iv) Will this compound be a good conductor of electricity? (2017)
 View Answer
View Answer 
Ans: (i) The type of bond in compound Z is an ionic bond.
(ii) Ionic compounds generally have high melting and boiling points due to the strong electrostatic forces of attraction between the positive and negative ions.
(iii) Ionic compounds like compound Z do not dissolve in non-polar solvents like kerosene or petrol. They are only soluble in polar solvents.
(iv) No, compound Z will not be a good conductor of electricity in a solid state because the ions are held in a fixed position and cannot move. However, it may conduct electricity when dissolved in water or molten state as the ions become free to move and carry electric charge.
Q15: Design an activity to show that metals are good conductors of heat and have high melting points.
 View Answer
View Answer 
Ans:
Activity for Conductivity:
- Take a metal rod (e.g., copper) and fix thumbtacks with wax at equal intervals.
- Heat one end with a burner.
Observation: Wax melts sequentially from the heated end, showing heat conduction.
Activity for Melting Point:
Heat small pieces of copper and plastic in separate crucibles.
Observation: Plastic melts quickly; copper requires much higher heat, indicating a high melting point.
Explanation:
- Metals conduct heat due to free electrons.
- High melting points result from strong metallic bonds.
Q16: (a) Name the following: (CBSE 2020)
(i) Metal that can be cut by a knife
(ii) Lustrous non-metal
(iii) Metal that exists in liquid state at room temperature
(iv) Most malleable and ductile metal
(v) Metal that is the best conductor of electricity
(vi) Non-metal that can exist in different forms
(b) How are alloys better than metals? Give the composition of solder and amalgam.
 View Answer
View Answer 
Ans: (a) (i) Sodium
(ii) Iodine
(iii) Mercury
(iv) Gold
(v) Silver
(vi) Carbon
(b) Alloys offer several advantages over pure metals:
- They are generally stronger.
- They resist corrosion better.
- They often have lower melting points.
- They usually have lower electrical conductivity.
The composition of: - Solder: An alloy of lead and tin.
- Amalgam: An alloy of mercury with another metal.
Q17: Some metals react with acids to produce salt and hydrogen gas. Illustrate it with an example. How will you test the presence of this gas? (2024)
 View Answer
View Answer 
Ans: Zn + H2SO4 → ZnSO4 + H2 (g)
To test for the presence of hydrogen gas:
- Bring a burning matchstick near the gas.
- If hydrogen is present, it will burn with a pop sound.
Q18: Compare in tabular form the reactivities of the following metals with cold and hot water: (2020)
(a) Sodium
(b) Calcium
(c) Magnesium
 View Answer
View Answer 
Ans: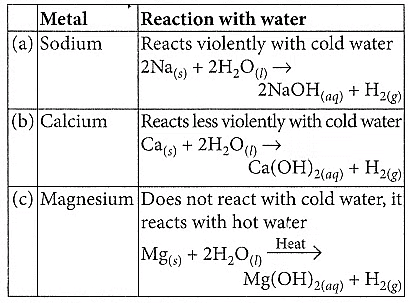
Important Topics for Preparation
Based on the analysis, the following topics are critical for CBSE Class 10 students preparing for "Metals and Non-metals":
1. Reactivity Series and Displacement Reactions
- The reactivity series arranges metals in decreasing order of reactivity:
K > Na > Ca > Mg > Al > Zn > Fe > Pb > Cu > Ag > Au. - In displacement reactions, a more reactive metal displaces a less reactive one from its salt solution, e.g., Zn + CuSO₄ → ZnSO₄ + Cu.
- Observations include color changes (CuSO₄ blue to FeSO₄ pale green) and deposition of reddish-brown copper. This series helps predict reaction outcomes and arrange metals by reactivity.
Focus: Memorize the series, predict displacement reactions, and note observations like color changes and hydrogen gas evolution.
2. Extraction and Refining of Metals
Different methods are used based on reactivity:
Electrolytic reduction for highly reactive metals (Na, Mg, Al).
Carbon reduction for moderately reactive metals (Fe, Zn).
Thermite process where Al reduces Fe₂O₃ to Fe.
Common ores: cinnabar (HgS), bauxite (Al₂O₃), and zinc carbonate (ZnCO₃). Key processes include roasting (sulphides → oxides), calcination (carbonates → oxides), and reduction. Refining is often done by electrolysis (CuSO₄ solution for copper).
Focus: Learn the sequence—concentration, roasting/calcination, reduction, and refining—along with important equations.
3. Corrosion and Its Prevention
- Corrosion is the reaction of metals with air and moisture. Rusting of iron requires both water and oxygen:
4Fe + 3O₂ + 2nH₂O → 2Fe₂O₃·nH₂O. - Other metals also corrode: copper forms a green patina (Cu₂(OH)₂CO₃), silver forms black Ag₂S, and aluminum forms a protective Al₂O₃ coating.
- Prevention methods include galvanization, painting, oiling, and alloying.
Focus: Remember the conditions for rusting, corrosion products, and prevention methods.
4. Ionic Compounds and Bonding
- Ionic bonds form by electron transfer, e.g., MgCl₂ forms when Mg loses two electrons and two Cl atoms gain one each. Compounds like MgCl₂, CaO, Na₂O, and AlN are typical examples.
- Properties: high melting/boiling points, water solubility, electrical conductivity in molten/aqueous state, and brittleness. Cations include Mg²⁺, Na⁺; anions include Cl⁻, O²⁻.
Focus: Practice electron-dot diagrams and memorize ionic properties.
5. Amphoteric Oxides
Amphoteric oxides react with both acids and bases. Examples: Al₂O₃, ZnO.
With acids: Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O.
With bases: Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O.
ZnO behaves similarly.
Focus: Learn common amphoteric oxides and their dual reactions.
6. Properties of Metals and Non-Metals
- Metals are malleable, ductile, good conductors, and usually form basic oxides. They react with acids to give hydrogen and with water (Na, Ca, etc.) to form hydroxides.
- Non-metals are brittle, poor conductors, and form acidic oxides such as SO₂.
- Special cases: mercury is a liquid metal; gold and silver are highly malleable and ductile.
Focus: Compare metals and non-metals in tabular form with examples.
7. Alloys
- Alloys are mixtures of metals (sometimes with non-metals) made to enhance properties.
- Examples: brass (Cu + Zn), solder (Pb + Sn), amalgam (Hg + another metal).
- They provide strength, resistance to corrosion, and sometimes lower melting points.
Focus: Memorize common alloy compositions and their uses.
8. Reactions with Acids and Water
- Metals above hydrogen in the reactivity series react with acids to release H₂ gas (e.g., Zn + H₂SO₄ → ZnSO₄ + H₂). With nitric acid, hydrogen is usually not evolved because it oxidizes to water.
- Metals like Na, K, and Ca react vigorously with cold water, producing hydroxides and hydrogen. H₂ gas is confirmed by the pop test with a burning matchstick.
Focus: Revise acid–metal reactions, exceptions, and gas tests.
9. Non-Metals and Their Compounds
Non-metals include carbon (allotropes), sulfur (forms SO₂), and bromine (a liquid). Compounds include:
CO₂ + H₂O → H₂CO₃ (carbonic acid).
S + O₂ → SO₂ (acidic gas).
They form acidic oxides, are brittle, and are poor conductors.
Focus: Memorize key reactions and properties of common non-metals.
Study Tips
Memorize: reactivity series, extraction steps, corrosion products, ionic properties.
Practice: balancing equations, predicting displacements, solving assertion–reason questions.
Focus on: thermite reaction, rusting, ionic bonding, refining of copper and aluminum.
Revise NCERT activities: rusting setup, electrolytic refining, metal–acid reactions.
Diagrams: electron-dot structures and refining apparatus.
FAQs on Sure Shot Questions for Board Exams: Metals & Non-metals - Class 10
| 1. What are the main differences between metals and non-metals? |  |
| 2. What are some common properties of metals? |  |
| 3. How do non-metals react with metals? |  |
| 4. What are some applications of metals and non-metals in daily life? |  |
| 5. What is the significance of the reactivity series in metals? |  |













