Based on the analysis, the following topics are critical for CBSE Class 10 students preparing for "Carbon and its Compounds":
Class 10 Exam > Class 10 Notes > Sure Shot Questions for Board Exams: Carbon & its Compounds
Sure Shot Questions for Board Exams: Carbon & its Compounds - Class 10 PDF Download
| Table of contents |

|
| Introduction |

|
| Key Questions |

|
| Important Topics for Preparation |

|
| Study Tips |

|
Introduction
The CBSE Class 10 chapter "Carbon and Its Compounds" is pivotal, covering hydrocarbons, functional groups, covalent bonding, and key reactions like hydrogenation and esterification. Analysis of previous year question papers (2015–2025) reveals recurring themes such as homologous series, ethanol reactions, and micelle formation, which dominate exams due to their syllabus weightage. Based on these trends, we’ve compiled a list of high-probability questions for the upcoming exam, grounded in their frequency and CBSE’s question-framing patterns.
Here’s a chart showing the frequency of key topics in CBSE Class 10 "Carbon and Its Compounds" based on previous year paper analysis: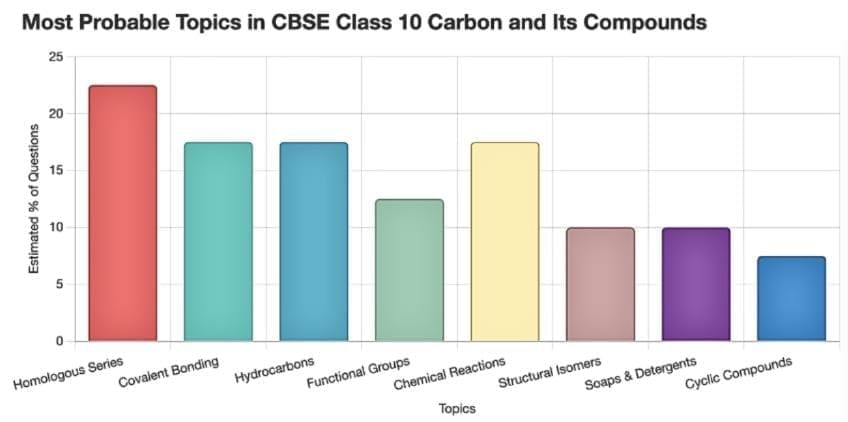
Key Questions
Q1: The structural formula of Cyclohexane is (1 Mark) (CBSE 2024)
(a) 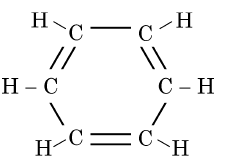
(b) 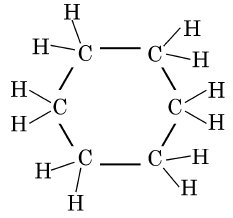
(c) 
(d) 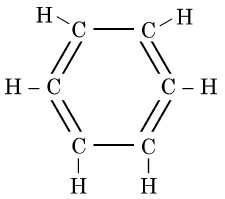
 View Answer
View Answer 
Ans: (b)
The correct structural formula for cyclohexane is shown in option (b). Cyclohexane, C6H12 , is a cyclic hydrocarbon with a six-membered ring structure, where each carbon atom is bonded to two other carbon atoms and two hydrogen atoms. This configuration ensures all carbon-carbon bonds are single, fitting the description and structure in option (b).
Q2: (A) (a) What is meant by the term homologous series of carbon compounds? Write molecular formula of any two consecutive members of homologous series of ketones.
(b) Write chemical equation of the reactions of ethanoic acid with (i) Sodium hydroxide and (ii) Ethanol (in the presence of an acid); giving the name of the products in each case.
(c) Draw the structure of the molecule of benzene.
 View Answer
View Answer 
Ans: (A)
(a) Homologous Series: Series of compounds with the same functional group, differing by CH₂ unit.
- Ketones : Propanone (C₃H₆O), Butanone (C₄H₈O).
(b) Reactions:
(i) CH₃COOH + NaOH → CH₃COONa (sodium ethanoate) + H₂O
(ii) CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ (ethyl ethanoate) + H₂O
(c) Benzene Structure:
C₆H₆ (ring with alternating double bonds)Explanation:
- Ketones differ by CH₂; ethanoic acid forms a salt with NaOH, ester with ethanol.
- Benzene has a cyclic structure with delocalized electrons.
Q3: "Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration.” Give reasons to justify this statement. (Term II, 2021)
 View Answer
View Answer 
Ans: Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration. Carbon has an atomic number of 6, meaning it has four electrons in its outer shell. Here are the reasons why it prefers sharing electrons:
- If carbon were to gain four electrons, it would form a C4- anion. This would create strong repulsions among the eight electrons in the valence shell, making it hard for the nucleus to hold onto ten electrons.
- If it lost four electrons, it would create a C4+ cation. This process requires a significant amount of energy, as the nucleus would then only hold onto two electrons.
To overcome these challenges, carbon shares its valence electrons with other atoms. This sharing leads to the formation of a covalent bond, allowing both atoms to achieve a stable noble gas configuration.
Q4: Carbon forms compounds mainly by covalent bonding. Why? (Term II, 2021-22 C)
 View Answer
View Answer 
Ans: Carbon can form only covalent compounds because carbon can neither gain nor lose four electrons to acquire stable octet. The only way by which it can acquire the nearest noble gas configuration is by sharing its valence electrons with other C-atoms or atoms of other elements. Hence, carbon forms compounds mainly by covalent bonding.
Q5: Draw the electron dot structure of the molecules of (a) Oxygen, and (b) Nitrogen. The atomic numbers of oxygen and nitrogen are 8 and 7 respectively. (Term II, 2021-22 C)
 View Answer
View Answer 
Ans: (a) Formation of oxygen molecule: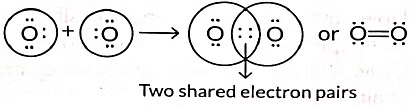
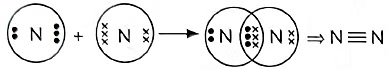
(b) Formation of nitrogen molecule: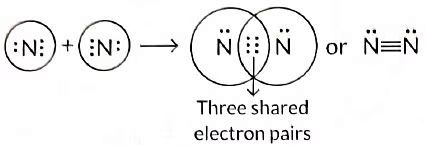
Q6: (a) Draw the electron dot structure for ethyne. (Term II, 2021-22)
(b) List two differences between the properties exhibited by covalent compounds and ionic compounds.
 View Answer
View Answer 
Ans: 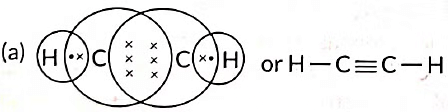
(b) 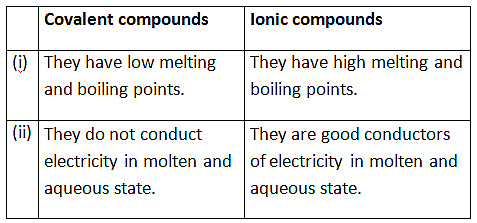
Q7: Draw two different possible structures of a saturated hydrocarbon having four carbon atoms in its molecule. What are these two structures of the hydrocarbon having same molecular formula called? Write the molecular formula and the common name of this compound. Also write the molecular formula of its alkyne. (Term II, 2021-22)
 View Answer
View Answer 
Ans: 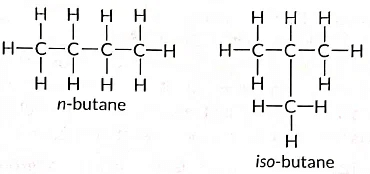
These are called structural isomers as they have the same molecular formula i.e., C4H10 but different structures. As the molecular formula is C4H10, common name of this compound is butane. The alkyne of four carbon atoms is butyne. Its structure i is as follows: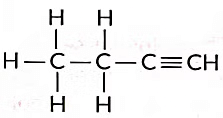
The molecular formula of butyne is C4H6.
Q8: (i) Draw two structural isomers of butane.
(ii) Draw the structures of propanol and propanone.
(iii) Name the third homologue of:
(a) alcohols
(b) aldehydes
(iv) Name the following: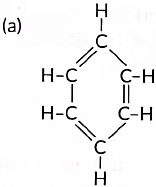
(b) CH3 - CH2CH = CH2
(v) Show the covalent bond formation in nitrogen molecule. (5 Marks) (2023)
 View Answer
View Answer 
Ans: (i) Structural isomers of butane are the following: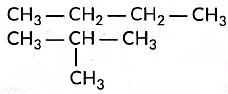
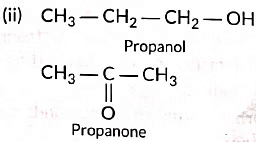
(iii) (a) Three homologue of alcohol are the following:CH3OH, CH3CH2OH, CH3CH2CH2OH
Third homologue of alcohol is CH3CH2CH2OH
(b) Three homologue of aldehyde are the following:
HCHO, CH3CHO, CH3CH,CHO
Third homologue of aldehyde is CH3CH2CHO.
(iv) (a) Benzene (b) But-1-ene
(v) Z = 7, 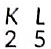
Q9: Covalent compounds are generally poor conductors of electricity. Why? (2020)
 View Answer
View Answer 
Ans: Covalent compounds are generally poor conductors of electricity due to the following reasons:
- Covalent compounds do not contain ions.
- They consist of atoms that share electrons, rather than transferring them.
- This lack of charged particles means they cannot conduct electricity effectively.
Q10: (A) A carbon compound 'A' on heating with excess conc. H₂SO₄ forms a compound 'B', which on addition of one mole of hydrogen gas in the presence of nickel catalyst forms a compound 'C'. 'C' on combustion in air forms 2 moles of carbon dioxide and 3 moles of water. Identify 'A', 'B' and 'C' and write their structures. Give chemical equations of the reactions involved. Also state the role of concentrated sulphuric acid in the formation of 'B' from 'A'.
OR
(B) A carbon compound 'A' is widely used as a preservative in pickles and has a molecular formula C₂H₄O₂. This compound reacts with ethanol to form a sweet smelling compound 'B'.
(i) Identify the compound 'A' and write its structure.
(ii) Write the chemical equation for the reaction of 'A' with ethanol to form compound 'B'. State the role of presence of an acid in the reaction.
(iii) How can we get compound 'A' back from 'B'?
(iv) How can 'A' be obtained from ethanol?
(v) Name the gas produced when compound 'A' reacts with washing soda.
 View Answer
View Answer 
Ans: (A)
A: Ethanol (C₂H₅OH); B: Ethene (C₂H₄); C: Ethane (C₂H₆).
Structures:
- Ethanol: CH₃–CH₂–OH
- Ethene: CH₂=CH₂
- Ethane: CH₃–CH₃
Equations:
- C₂H₅OH → C₂H₄ + H₂O (conc. H₂SO₄, 443 K)
- C₂H₄ + H₂ → C₂H₆ (Ni catalyst)
- C₂H₆ + 7/2 O₂ → 2CO₂ + 3H₂O
Role of H₂SO₄: Dehydrating agent, removes water from ethanol.
Explanation:
Ethanol dehydrates to ethene, which hydrogenates to ethane, confirmed by combustion products.
Q11: Write chemical equation to show what happens when ethanol:
(I) Burns in oxygen/air.
(II) Is heated at 443 K in excess conc. H₂SO₄.
(III) Reacts with acidified potassium dichromate.
 View Answer
View Answer 
Ans:
Equations:
- (I) C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O (Combustion)
- (II) C₂H₅OH → C₂H₄ + H₂O (Dehydration, conc. H₂SO₄, 443 K)
- (III) C₂H₅OH + [O] → CH₃CHO + H₂O (Oxidation to ethanal, acidified K₂Cr₂O₇)
Explanation:
- Butene isomers differ in double bond position.
- Ethanol burns to form CO₂ and water, dehydrates to ethene, and oxidizes to ethanal.
Q12: (a) Carry out following conversions:
(i) Ethanol to ethene
(ii) Ethanol to ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each. (2020)
 View Answer
View Answer 
Ans: (a) (i) When ethanol is heated with cone. H2SO4 at 443 K, ethene is obtained due to dehydration of ethanol.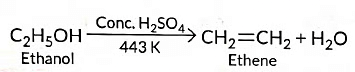
(ii) When 5 % alkaline KMn04 solution is added drop by drop to warm ethanol then it gets oxidised to ethanoic acid.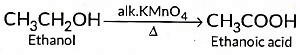
(b) Addition reactions: Those reactions in which atoms or group of atoms are simply added to a double or triple bond without the elimination of any atom or molecule, are known as addition reactions.
Substitution reactions: The reactions which involve the displacement or substitution of an atom or a group of atoms in an organic compound by another atom or group of atoms, are known as substitution reactions. Saturated hydrocarbons are fairly unreactive and inert in the presence of most of the reagents. However, in presence of sunlight, hydrocarbons undergo rapid substitution reactions. e.g.,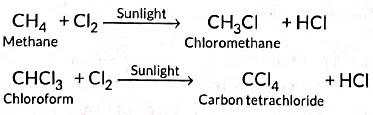
Q13: (a) A compound 'X' undergoes addition reaction with H2 to form a compound Y having molecular mass 30 g mol-1. ‘X’ decolorises bromine water and burns with a smoky flame. Identify ‘X’ and Y and write chemical equations of the reactions involved.
(b) Write the structural formulae of (i) Butanone, and (ii) Pentanoic acid.
(c) Would you be able to check if water is hard by using a detergent ? Give reason to justify your answer. (2020 C)
 View Answer
View Answer 
Ans: (a) As the molecular mass of Y is 30 g mol-1, it is ethane (C2H6 = 12 x 2 + 6 x 1 = 30).
‘X’ is ethene (CH2 = CH2) which decolourises Br2 water and burns with a smoky flame.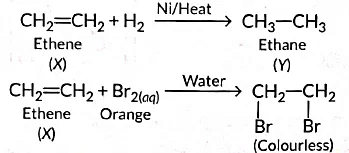
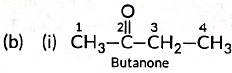
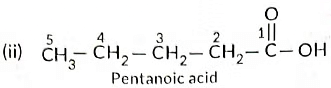
(c) No, you cannot determine if water is hard using a detergent. This is because detergents function effectively in both hard and soft water. The calcium and magnesium salts of detergents are soluble, allowing them to work well even in hard water.
Q14: When ethanol reacts with ethanoic acid in the presence of cone. H2SO4, a substance with fruity smell is produced. Answer the following:
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of cone. H2SO4 in this reaction. (Delhi 2016)
 View Answer
View Answer 
Ans: (i) When ethanol reacts with ethanoic acid in the presence of concentrated H₂SO₄, the product formed is ethyl ethanoate, which belongs to the class of ester compounds known for their fruity smell.
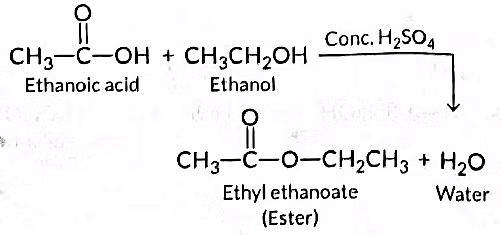
(ii) The role of concentrated H₂SO₄ in this reaction is two fold:
- It acts as a dehydrating agent, helping to remove water.
- It serves as a catalyst to speed up the reaction.
Q15: (a) Compare soaps and detergents on the basis of their composition and cleansing action in hard water.
(b) What happens when ethanol is treated with sodium metal? State about the behaviour of ethanol in this reaction.
(c) Name the following compound. (2018)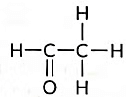
 View Answer
View Answer 
Ans: (a)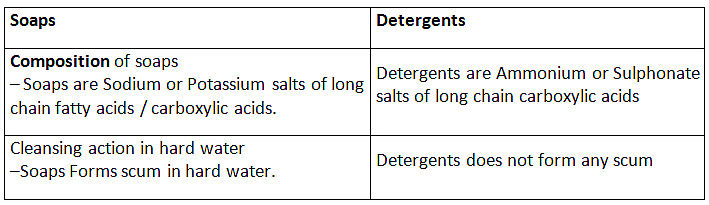
(b) Reaction of Ethanol with Sodium Metal:
- When ethanol reacts with sodium metal, hydrogen gas is released.
- Ethanol behaves like an acid in this reaction.
(c) Name of the compound is Ethanal and it is an Acetaldehyde
Q16: Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps. (Delhi 2017, Al 2015)
 View Answer
View Answer 
Ans: The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids.
Difference between Soap and Detergent
Soaps:
(i) Soaps are sodium salts of long chain carboxylic acids.
(ii) The ionic group in soap is -COO-Na+.
(iii) Soaps are not useful when water is hard.
Detergents
(i) Detergents are the odium salt of long chain benzene sulphonic acids.
(ii) The ionic groups in detergents is SO3-Na+ or SO4-Na+.
(iii) Detergent can be used for washing purpose even when water is hard
Cleansing action of soap can be described as follows:
The soap molecule is generally represented as RCOONa. In solution, it ionises to form RCOO- and Na+. Each soap molecule has a polar head group (carboxylate ion, COO- group) and a long nonpolar hydrocarbon tail (R group from long chain fatty acid). The polar head attracts the polar water molecule and is called hydrophilic end and the nonpolar tail attracts the water-insoluble oily or greasy dirt particles.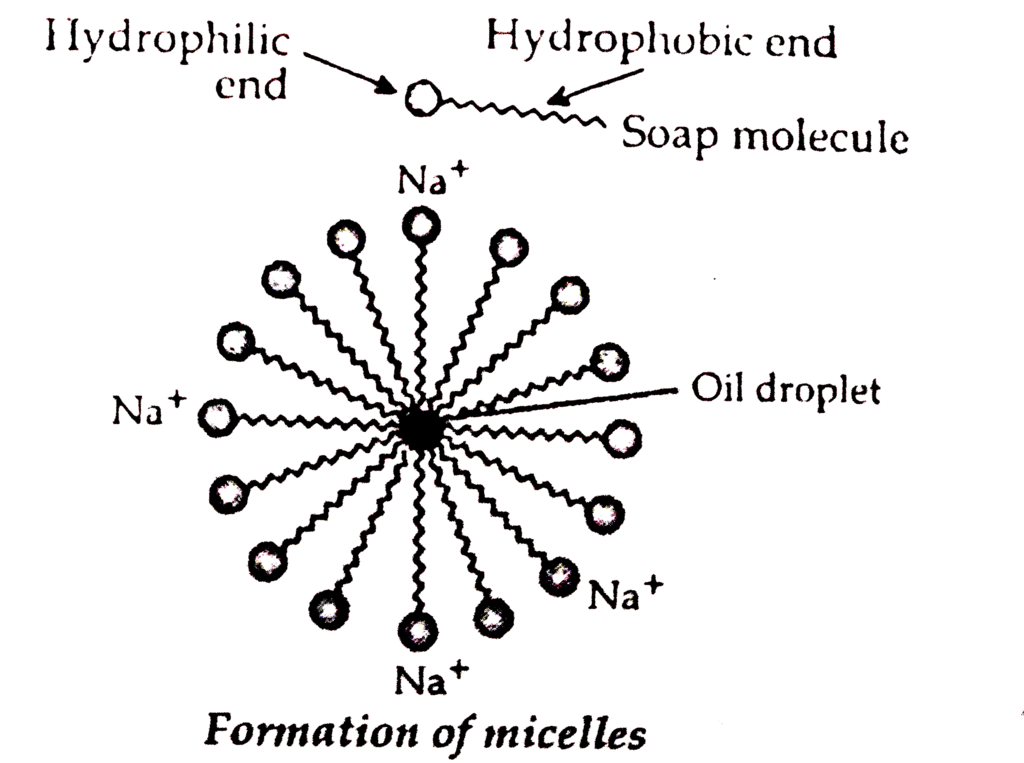
Q17: Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of the catenation of the two elements. Give reasons. (CBSE 2016, 11, 10)
 View Answer
View Answer 
Ans: Catenation is the ability of an atom to bond with other atoms of the same element. This property is notably exhibited by both carbon and silicon, but carbon demonstrates it to a far greater extent.
- Carbon can form long chains, branched structures, or rings due to its strong carbon-carbon bonds.
- Carbon has a valency of four, allowing it to bond with up to four other atoms, including other carbon atoms.
- This results in a vast number of stable compounds, as the bonds formed are strong and do not easily break.
- In contrast, silicon can form chains of only seven or eight atoms with hydrogen, but these compounds are highly reactive.
Overall, the unique ability of carbon to catenate leads to an extensive variety of compounds, making it essential for life and numerous applications.

Q18: (i) Define a homologous series of carbon compounds.
(ii) Why is the melting and boiling points of C4H8 higher than that of C3H6 or C2H4?
(iii) Why do we NOT see any gradation in the chemical properties of homologous series compounds?
(iv) Write the name and structures of (i) aldehyde and (ii) ketone with molecular form C3H6O.
 View Answer
View Answer 
Ans: (A) (i) A homologous series is a group of carbon compounds where each member differs from the next by a CH2 unit. They share the same functional group and exhibit similar chemical properties.
(ii) The melting and boiling points of C4H8 are higher than those of C3H6 or C2H4 because these points increase with molecular mass.
(iii) There is no gradation in the chemical properties of compounds in a homologous series because their properties are determined solely by their functional group, which remains constant.
(iv) (i) Aldehyde: Propanal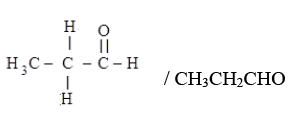
(ii) Ketone: Propanone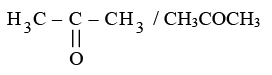
Important Topics for Preparation
1. Homologous Series
- A homologous series is a family of compounds with the same functional group, where successive members differ by –CH₂– (14 u).
- General formulas include: alkanes (CₙH₂ₙ₊₂), alkenes (CₙH₂ₙ), alkynes (CₙH₂ₙ₋₂), alcohols (CₙH₂ₙ₊₁OH), aldehydes (CₙH₂ₙO), carboxylic acids (CₙH₂ₙO₂).
- These compounds share similar chemical properties, while physical properties such as boiling and melting points increase with molecular mass.
- Examples are methane (CH₄) and ethane (C₂H₆) among alkanes, ethene (C₂H₄) and propene (C₃H₆) among alkenes, and ethanol (C₂H₅OH) and propanol (C₃H₇OH) among alcohols.
Focus: Memorize general formulas, practice identifying members (e.g., CH₃CHO, C₂H₅CHO), and understand trends in physical properties.
2. Covalent Bonding and Properties
- Carbon forms covalent bonds due to tetravalency (four valence electrons) and its inability to form stable C⁴⁺ or C⁴⁻ ions.
- Covalent compounds generally have low melting and boiling points (weak intermolecular forces), are poor conductors of electricity (no free ions), and include examples like methane (CH₄) and ethanol (C₂H₅OH).
- The property of catenation (self-linking) allows carbon to form long chains and rings, leading to structural diversity.
- Common electron-dot diagrams include Cl₂, NH₃, and ethyne.
Focus: Understand why carbon favors covalent bonding, revise properties, and practice electron-dot structures.
3. Hydrocarbons
Hydrocarbons can be saturated or unsaturated.
Saturated hydrocarbons (alkanes) have only single bonds, e.g., butane (C₄H₁₀), and burn with a clean flame.
Unsaturated hydrocarbons (alkenes and alkynes) contain double or triple bonds, e.g., ethene (C₂H₄) and ethyne (C₂H₂), and often produce a sooty flame.
Tests for unsaturation include the flame test (sooty flame) and bromine water test (decolorization).
For instance, C₇H₁₄ is identified as an alkene, and ethene’s structure is commonly asked.
Focus: Differentiate saturated from unsaturated hydrocarbons, recall combustion products, and practice structural drawings.
4. Functional Groups and Nomenclature
- Important functional groups include alcohol (–OH), aldehyde (–CHO), carboxylic acid (–COOH), halogen (–Br), and ketone (–C=O).
- Examples of nomenclature: ethanol (C₂H₅OH), ethanoic acid (CH₃COOH), propanal (C₃H₆O), and butanone (C₄H₈O).
Focus: Memorize functional groups, learn systematic naming rules, and draw basic structures.
5. Chemical Reactions of Carbon Compounds
Key reactions include:
Hydrogenation: Unsaturated to saturated hydrocarbons, e.g., C₂H₄ + H₂ → C₂H₆ (Ni catalyst).
Oxidation: Ethanol to ethanoic acid using KMnO₄ or K₂Cr₂O₇.
Esterification: Ethanol + ethanoic acid → ethyl ethanoate.
Saponification: Ester + NaOH → alcohol + carboxylate salt.
Substitution: Methane reacts with chlorine in sunlight to form chloromethane and HCl.
Conditions to note: concentrated H₂SO₄ acts as a dehydrating agent at 443 K; catalysts like Ni or oxidizing agents like KMnO₄ are essential in specific reactions.
Focus: Learn balanced equations, reaction conditions, and products.
6. Structural Isomers
- Isomers have the same molecular formula but different structural arrangements.
- Examples include butane (n-butane and isobutane) and butene (1-butene and 2-butene).
Focus: Practice drawing isomers of C₄H₁₀ and C₄H₈ and identifying structural differences.
7. Soaps and Detergents
- Soaps are sodium or potassium salts of long-chain carboxylic acids, while detergents are synthetic compounds effective even in hard water.
- Soaps form micelles, where hydrophobic tails trap grease/dirt and hydrophilic heads remain in water.
- In hard water, soaps form scum with calcium and magnesium ions, while detergents work efficiently.
Focus: Understand micelle formation, cleansing action, and differences between soap and detergent.
8. Cyclic Compounds
- Examples include cyclohexane (C₆H₁₂) with single bonds and benzene (C₆H₆) with alternating double bonds.
- Benzene contains 9 single and 3 double bonds.
Focus: Memorize benzene and cyclohexane structures and bond types.
Study Tips
Memorize: General formulas of homologous series, functional groups, and important reaction equations.
Practice: Electron-dot diagrams, balanced equations, structural isomers, and nomenclature.
Revise NCERT Activities: Ethanol oxidation, flame test for hydrocarbons, and micelle formation.
Prioritize for exams: Homologous series, ethanol reactions (oxidation, esterification), and soap vs. detergent.
FAQs on Sure Shot Questions for Board Exams: Carbon & its Compounds - Class 10
| 1. What are the main characteristics of carbon and its compounds? |  |
Ans. Carbon is a non-metal that has unique properties, allowing it to form a vast number of compounds. Some key characteristics include its ability to form four covalent bonds, leading to a variety of molecular structures such as chains and rings. Carbon compounds can be classified into organic (containing carbon-hydrogen bonds) and inorganic (not primarily based on carbon). They exhibit diverse physical and chemical properties, such as varying boiling and melting points, solubility, and reactivity based on their structure and functional groups.
| 2. What are some common uses of carbon compounds in daily life? |  |
Ans. Carbon compounds are integral to many aspects of daily life. For example, hydrocarbons such as gasoline and diesel are used as fuels, while carbon-based materials like plastics (polymers) are found in numerous products, from containers to clothing. Additionally, carbon compounds like glucose (C₆H₁₂O₆) are vital for energy in biological systems, and many pharmaceuticals contain carbon-based structures, highlighting their importance in healthcare.
| 3. How do carbon compounds contribute to environmental issues? |  |
Ans. Carbon compounds play a significant role in environmental issues, particularly in the context of climate change and pollution. For instance, carbon dioxide (CO₂) and methane (CH₄) are greenhouse gases that contribute to global warming. The burning of fossil fuels releases these gases into the atmosphere, leading to climate change. Additionally, certain carbon compounds can result in air and water pollution, affecting ecosystems and human health.
| 4. What is the significance of functional groups in carbon compounds? |  |
Ans. Functional groups are specific groups of atoms within molecules that determine the chemical reactivity and properties of carbon compounds. They play a crucial role in organic chemistry, influencing the behavior of molecules during chemical reactions. For example, hydroxyl groups (-OH) make alcohols polar and soluble in water, while carboxyl groups (-COOH) confer acidity to carboxylic acids. Understanding functional groups is essential for predicting the reactions and interactions of organic molecules.
| 5. What are isomerism and its significance in carbon compounds? |  |
Ans. Isomerism refers to the phenomenon where compounds have the same molecular formula but differ in the arrangement of atoms, leading to distinct properties. There are two main types: structural isomers, which differ in connectivity, and stereoisomers, which have the same connectivity but differ in spatial arrangements. Isomerism is significant in carbon compounds as it can result in vastly different chemical behaviors and properties, which is crucial in fields such as pharmaceuticals, where the efficacy of a drug can depend on its isomeric form.
Related Searches













