Unit Test (Solutions): A Journey through States of Water | Science for Class 6 PDF Download
Attempt all questions.
Time: 1 hour
M.M. 30
- Question numbers 1 to 7 carry 1 mark each.
- Question numbers 8 to 12 carry 2 marks each.
- Question numbers 13 to 15 carry 3 marks each.
- Question number 16 carries 4 marks each.
Q1: Which of the following is a state of water that is hard to touch and can be held in hands? (1 Mark)
(i) Liquid
(ii) Gas
(iii) Solid
(iv) Vapour
Ans: (iii) Solid
Ice is the solid state of water, which feels hard and can be held, unlike liquid water that flows away.
Q2: The process by which water changes from liquid to vapour at room temperature is called __________. (1 Mark)
Ans: evaporation
Evaporation is the conversion of liquid water into water vapour, as seen in drying puddles or wet clothes.
Q3: Water droplets forming on the outer surface of a cold glass tumbler is an example of __________. (1 Mark)
(i) Evaporation
(ii) Freezing
(iii) Condensation
(iv) Melting
Ans: (iii) Condensation
Condensation occurs when water vapour in the air contacts a cold surface and turns into liquid droplets.
Q4: Which property of water vapour differs from ice and liquid water? (1 Mark)
(i) It flows but does not spread
(ii) It spreads out in the entire available space
(iii) It retains a fixed shape
(iv) It takes the shape of the container only
Ans: (ii) It spreads out in the entire available space
Water vapour, in the gaseous state, diffuses to fill all available space, unlike ice or liquid water.
Q5: The cooling effect in an earthen pot (matka) is due to __________. (1 Mark)
(i) Seeping of water into the ground
(ii) Evaporation of water seeping through the pot
(iii) Freezing of water inside the pot
(iv) Condensation on the pot's surface
Ans: (ii) Evaporation of water seeping through the pot
Evaporation of seeping water absorbs heat, cooling the water inside the earthen pot.
Q6: Clouds form when water vapour turns into tiny droplets around __________. (1 Mark)
Ans: dust particles
Water vapour condenses around dust particles in cool air to form cloud droplets.
Q7: In the water cycle, the process that brings water back to Earth's surface as rain or snow is __________. (1 Mark)
(i) Evaporation
(ii) Condensation
(iii) Precipitation
(iv) Freezing
Ans: (iii) Precipitation
Precipitation occurs when heavy cloud droplets fall as rain, snow, or hail.
Q8: Explain the difference in shape between ice (solid) and liquid water. (2 Marks)
Ans: Ice retains its own fixed shape regardless of the container, as solids have a definite shape. Liquid water takes the shape of the container it is poured into, as liquids flow and adapt to the container's form.
Q9: Why does water evaporate faster from a plate than from a bottle cap? (2 Marks)
Ans: Water evaporates faster from a plate because it has a larger exposed surface area in contact with air, allowing more water molecules to escape as vapour compared to the smaller area in a bottle cap.
Q10: Describe how the mass changes when a cold glass tumbler with ice cubes is left undisturbed. (2 Marks)
Ans: The measurable mass increases over time because water vapour from the air condenses on the cold outer surface, forming liquid droplets. The glass and ice themselves do not gain mass, but the added water droplets increase the total mass of the system.
Q11: Give two examples of evaporation from daily life and one factor affecting its speed. (2 Marks)
Ans: Examples include drying wet clothes and sweat evaporating from the body. One factor is temperature; evaporation is faster on a hot day as heat provides energy for water molecules to become vapour.
Q12: How does wind affect the evaporation of water? (2 Marks)
Ans: Wind increases evaporation by moving air over the water surface, carrying away saturated air (with high water vapour) and replacing it with drier air, allowing more evaporation to occur.
Q13: Compare the ability to flow and spread in the three states of water. (3 Marks)
Ans:
| State of Water | Ability to Flow | Ability to Spread |
|---|---|---|
| Ice (Solid) | Does not flow, retains a fixed shape | Does not spread, keeps its own shape |
| Liquid Water | Flows easily and takes the shape of the container | Spreads on a surface but keeps its volume constant |
| Water Vapour (Gas) | Flows freely | Spreads out to completely fill the available space |
Q14: Explain the role of condensation in cloud formation and rain, including the importance of dust particles. (3 Marks)
Ans: Condensation turns water vapour into tiny droplets when warm air rises and cools at higher altitudes. These droplets form around dust particles, which act as nuclei, leading to cloud formation. As more droplets combine, they grow larger and heavier until they fall to the ground as rain. Sometimes, under very cold conditions, the condensed water may fall as hail or snow. This process helps return evaporated water to Earth, completing the water cycle.
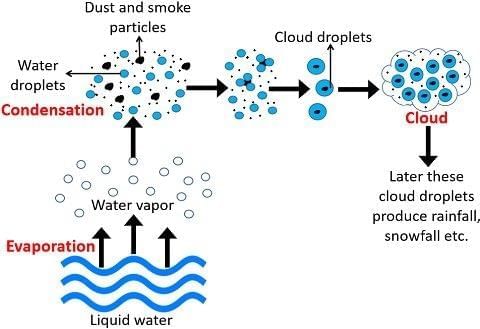
Q15: Describe how to change water from liquid to solid and then to gas, naming the processes involved. (3 Marks)
Ans:
| Step | Process (Name) | State Change | Example / Explanation |
|---|---|---|---|
| 1 | Freezing | Liquid → Solid | Cool liquid water in a freezer to form ice. |
| 2 | Melting | Solid → Liquid | Heat the ice so it melts and turns back into water. |
| 3 | Evaporation / Boiling | Liquid → Gas | Continue heating the water until it changes into water vapour. |
Note: These state changes are reversible — cooling changes gases back to liquids (condensation) and liquids back to solids (freezing). Similar state changes can be seen in substances like wax and coconut oil.
Q16: Discuss the factors that affect evaporation speed and explain the cooling effect caused by evaporation, with examples. (4 Marks)
Ans: Several factors influence how fast water evaporates.
- Surface area is one major factor — water spread out in a plate evaporates faster than in a bottle cap because more water molecules are exposed to air.
- Temperature also affects evaporation — water evaporates faster on a hot sunny day than on a cold day because heat gives energy to water molecules to escape into the air.
- Wind speed plays an important role too — on a windy day, evaporation is faster as moving air removes the water vapour near the surface and replaces it with dry air.
- Humidity slows down evaporation because when the air already contains a lot of water vapour (as on rainy days), fewer water molecules can escape.
Evaporation produces a cooling effect because it absorbs heat from the surroundings. For example, water in an earthen pot stays cool as some water seeps through and evaporates. Sprinkling water on the floor or roof also cools the surface. Similarly, sweat or alcohol on the skin feels cool as it evaporates, taking away heat.
|
67 videos|282 docs|27 tests
|
FAQs on Unit Test (Solutions): A Journey through States of Water - Science for Class 6
| 1. What are the three main states of water and how do they differ from each other? |  |
| 2. How does temperature affect the states of water? |  |
| 3. What is the process of evaporation and how does it relate to the water cycle? |  |
| 4. Can water exist in more than one state at the same time? If so, how? |  |
| 5. What is the significance of understanding the states of water in our daily lives? |  |
















