Chemical Effects of Electric Current Class 8 Notes Science Chapter 11
| Table of contents |

|
| Introduction |

|
| Do liquids conduct electricity? |

|
| Chemical Effects of Electric Current |

|
| Electroplating |

|
| How a layer of one metal can be deposited on top of another? |

|
Introduction
Have you ever been told not to touch electrical appliances with wet hands?
This is important to know because some materials conduct electricity easily, while others do not. Metals like copper and aluminum are good conductors, while rubber and plastic are poor conductors. In earlier classes, we used a tester to check if materials conduct electricity.
Now, let’s explore whether liquids can conduct electricity too!
Conduction of Electricity
- Conduction of Electricity or Electrical Conduction means electrically charged particles moving through a material.
- This movement can create an electric current when influenced by an electric field.
- Just like some solids, certain liquids can also conduct electricity.
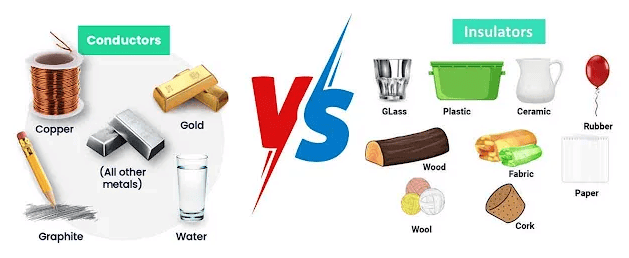
Conductor: Substances which allow electricity to pass through them are called conductors.
For example: Silver, gold, aluminium, copper, acidic solution, salt solution, etc.
Insulator: Substances which do not allow electric current to pass through them are called insulators
For example: Plastic, rubber, glass, air etc.
| Good Conductor | Bad Conductor |
| Substances which allow electric current to pass through them easily are called good conductors of electricity. | Some substances allows electric current to pass through them but in very little amount. Therefore, such substances called bad conductors of electricity, rather than being called as insulator. |
| For example : Silver, gold, aluminium, etc. | For example : Wood, paper, air, rubber, etc. |
 Good and Bad Conductors of Electricity
Good and Bad Conductors of Electricity
In fact, most of the substances allow electric current to pass through them under certain conditions, so instead of using terms conductors and insulators, good conductors and bad conductors are used.
Do liquids conduct electricity?
Yes, most liquids can conduct electricity because they have free electrons or ions that carry the electricity through the liquid. Solutions of acids, bases and salts in water conduct electricity and hence are called electrolytes.
For example- Lemon juice, sodium hydroxide and Tap water, Milk, Vinegar.
Whereas distilled water doesn’t conduct electricity as it doesn’t contain salts hence distilled water is a bad conductor of electricity. Oil, petrol and kerosene are poor conductors of electricity as they do not have free electrons or ions to conduct electricity.
Note – Milk is a good conductor of electricity because it contains water and lactic acids.
How conductivity of liquids can be tested?
Let us demonstrate the conduction of electricity in liquids and to test whether a given liquid is a conductor of electricity using a compass needle or LED by a simple experiment.
- For this, you need a compass needle, an empty matchbox, two electric cells, some wires, a plastic bottle cap, and the liquid which is to be tested.
- Keep the compass needle inside the empty tray of matchbox and wrap a couple of rubber bands around it.
- Connect the wires to the battery and insert two wires in the liquid which is kept in the plastic bottle cap.
- You will observe that when the current flows through the wire, there is deflection in the compass needle.
- This shows that the given liquid conducts electricity. In fact, magnetic compass needle can detect even feeble current.
- LED can also be used in place of compass needle.
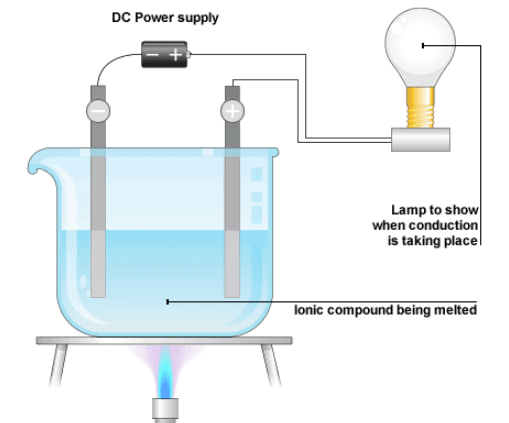 Testing of conduction of electricity through liquid
Testing of conduction of electricity through liquid
Heating Effect of Electric Current
- Heat is produced when an electric current flows through a conductor.
- This is referred to as the heating effect of electric current.
- The glowing of the bulb is caused by the heating effect of the current flowing through it.
 Glowing bulb
Glowing bulbTo determine if a substance conducts or not by the heating effect:
- When current is passed through the bulb, the filament (tungsten) heats up to a high temperature, causing the bulb to glow.
- However, if the current is extremely small, the filament will not be heated to a high temperature and will thus not glow as a result of this.
Question: LED bulbs are more suitable for testing the electrical conductivity of liquids. Why?
LED bulbs are better for testing the electrical conductivity of liquids because:
Low Current Needed: LED bulbs can glow even with a small amount of current. This makes them more useful for checking liquids that don't conduct electricity very well.
More Sensitive: LEDs can detect even a small flow of electricity through a liquid, making it easier to see if the liquid conducts electricity.
Saves Energy: LEDs use less power, which makes them a good option for experiments.
Safe to Use: Since LEDs work on low current, they are safer to use during experiments.
Regular bulbs need more electricity to glow, so they might not work with liquids that conduct electricity weakly. That's why LEDs are a better choice!
 LED light
LED light Chemical Effects of Electric Current
- The chemical effects of electric current can be defined as the passage of an electric current through a conducting solution or an electrolyte that causes chemical changes.
- This is because of chemical reactions that take place when an electric current passes through a solution.
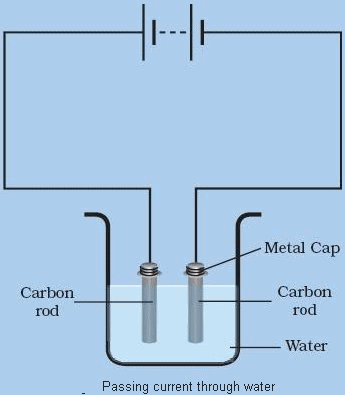 Chemical Effect of Electric current
Chemical Effect of Electric current
Applications of Chemical Effect of Electric Current
- Extraction of metals from their ores.
- Purification of metals
- Production of compounds.
- Decomposition of compounds.
Note- William Nicholson, was the first to discover the chemical effect of current. He discovered that if electrodes were immersed in water, and a current was passed they dissociate into hydrogen ions and oxygen ions.
Electrode: An electrode is a conductor of electricity that can carry electric current into non-metals and other poor conductors of electricity.
Electrolyte: The electrolyte can be defined as the liquids which allow an electric current to pass through them and split themselves on the passage of electric current.
Electrolysis: The production or occurrence of chemical change in an electrolyte when an electric current is passed through it. The process of electrolysis shows the following things Depending on the nature of the solution and the electrode.
- Release of gas bubbles on the electrodes.
- When current is conducted through water, oxygen and hydrogen bubbles are created.
- On the positive electrode, oxygen bubbles will be present, whereas hydrogen bubbles will be present on the negative electrode.
- Chemical reaction takes place when an electric current passes through a conducting solution.
- As a result, gas bubbles could form on the electrodes.
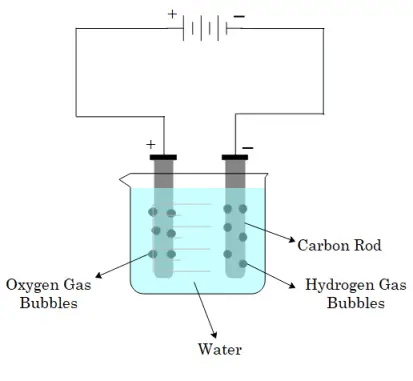
Release of bubbles when current passes through liquid
- Deposits of metal on electrodes.
- Change of colour of solution depending upon the electrodes.
Cathode and Anode
The electrode connected to the negative terminal of the battery is called the cathode (negative electrode).
The electrode connected to the positive terminal of the battery is called an anode (positive electrode).
Electroplating
The process by which a layer of metal is deposited over another metal by the passage of electric current. The kind of metal that is usually electroplated are gold, silver, tin, zinc, copper, chromium.
Reason for electroplating
Electroplating is a very useful process. It is widely used in industry. This technique for depositing a layer of metal. The deposited metal has desired properties. Electroplating is utilized in various industries.
The main reason is as follows.
- To protect the metal object by coating different metal on the metal object.
- Cost-efficient for example since chromium is a costly metal, the objects are made of a cheaper metal and a chromium coating is applied for a shiny appearance.
- Prevent metal from corrosion
Applications of electroplating
- Jewellery makers electroplate silver and gold on less expensive metals.
- Medical equipment is made up of nickel, which is harmful to the human body; hence, to avoid it from coming in contact with the body, a coating of platinum or gold is applied on the surface of the nickel.
- Tin cans used to store food are traditionally made of iron coated with tin. The coating of tin is used because it is corrosion-resistant and does not poison the food.
- Thus, the food is prevented from coming in contact with iron and getting spoilt.
- Iron is used in bridges and automobiles to provide strength; however, iron tends to corrode and rust.
- A coating of zinc is deposited on iron to protect it from corrosion and the formation of rust.
- Because of its shiny appearance, chromium plating is used for vehicle parts and bath fittings.
- Galvanization – Coating of zinc is deposited on iron to protect it from corrosion.
- Bridges and various parts of automobiles are made up of iron and are coated with zinc in order to prevent rust (Galvanization) because it provides strength.
 Use of Electroplating
Use of Electroplating
How electroplating can be done?
Electroplating is based on the chemical effects of electricity. Electroplating helps to prevent rusting.
To get the coating of a different metal the following is the process
- Two electrodes should be made of different metals.
- The metal on which coating is to be done should be used as a cathode while the metal to be deposited should be made the anode.
- The electrolyte should be a solution of the metal to be coated. For example - The coating of zinc over a copper object, the copper object is used as a cathode and a zinc plate as an anode and zinc sulphate as the electrolyte.
- The container or vessel consisting of the cathode, anode and electrolyte is called an electrolytic cell.
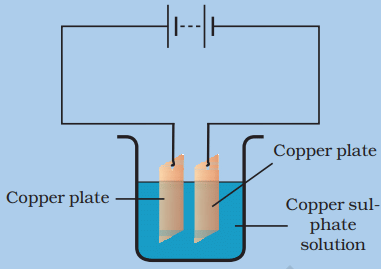
How a layer of one metal can be deposited on top of another?
- When an electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate.
- The free copper gets drawn to the electrode connected to the negative terminal (cathode) of the battery and gets deposited on it.
- The process of electroplating takes some time to complete.
- The time taken by the process depends upon the strength of the current and also on the concentration of the electrolyte.
- These two factors help to increase the speed of electroplating.
- We should ensure that the electrode is clean.
- The electrodes used are made up of different materials.
- One of the electrodes is made of the same metal as the electrolyte solution.
- The second electrode needs to be the material on which coating takes place.
|
92 videos|296 docs|44 tests
|
FAQs on Chemical Effects of Electric Current Class 8 Notes Science Chapter 11
| 1. Do all liquids conduct electricity? |  |
| 2. What are the chemical effects of electric current? |  |
| 3. What is electroplating and how does it work? |  |
| 4. Why is electroplating used in various industries? |  |
| 5. Can electroplating change the properties of the base metal? |  |
















