NEET Exam > NEET Notes > Chemistry Class 12 > NCERT Solutions: Coordination Compounds
NCERT Solutions Class 12 Chemistry Chapter 5 - Coordination Compounds
Page No.125 - Intext Questions
Q5.1: Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt(III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane−1,2−diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane−1,2−diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II) Q5.2: Write the IUPAC names of the following coordination compounds:
Q5.2: Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl
Ans: (i) Hexaamminecobalt(III) chloride
(ii) Pentaamminechloridocobalt(III) chloride
(iii) Potassium hexacyanoferrate(III)
(iv) Potassium trioxalatoferrate(III)
(v) Potassium tetrachloridopalladate(II)
(vi) Diamminechlorido(methylamine)platinum(II) chloride
Page No.128 - Intext Questions
Q5.3: Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
- K[Cr(H2O)2(C2O4)2]
- [Co(en)3]Cl3
- [Co(NH3)5(NO2)](NO3)2]
- [Pt(NH3)(H2O)Cl2]
Ans: Both geometrical (cis-, trans-) isomers for K[Cr(H2O)2(C2O4)2] can exist. Also, optical isomers for cis-isomer exist. Trans-isomer is optically inactive. On the other hand, cis-isomer is optically active.
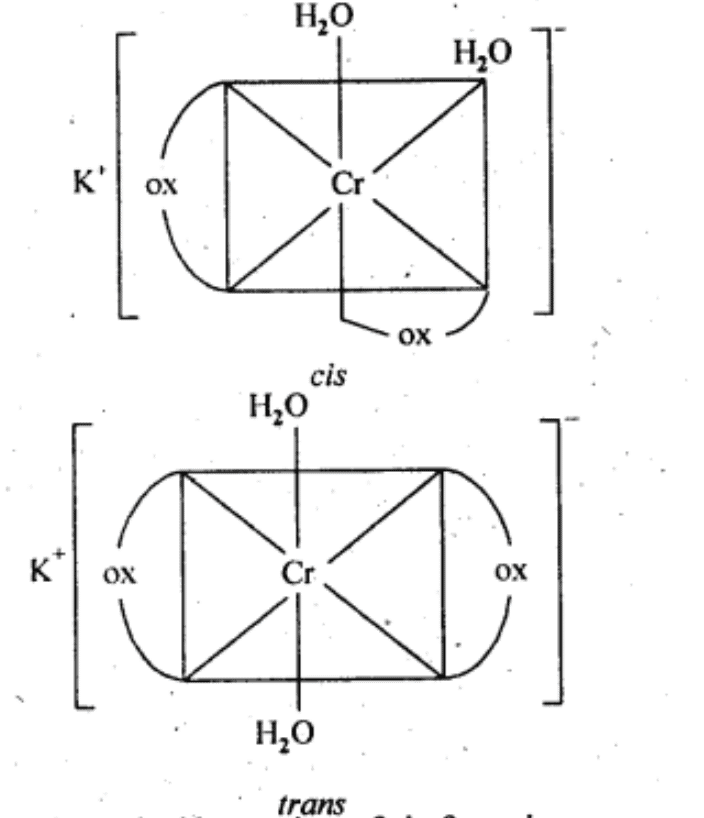
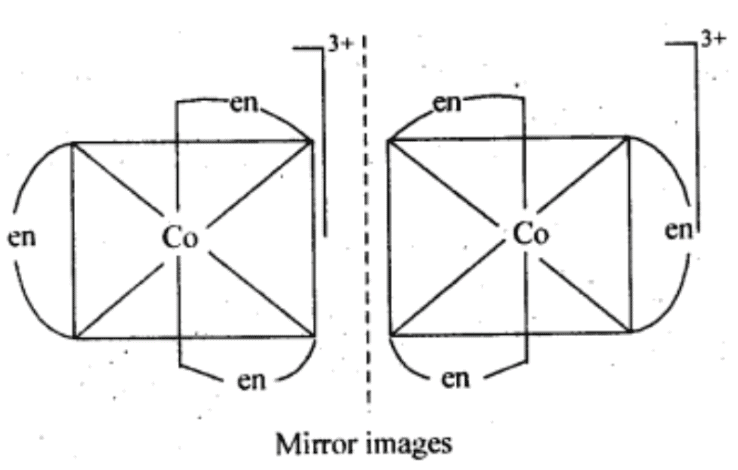
(ii) Two optical isomers for [Co(en)3]Cl3 exist.
(iii) [Co(NH3)5(NO2)](NO3)2]
A pair of optical isomers:
It can also show linkage isomerism.
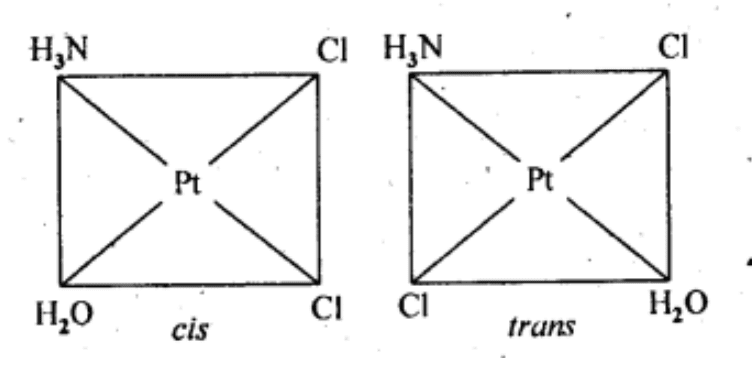
(iv) Geometrical (cis-, trans-) isomers of [Pt(NH3)(H2O)Cl2] can exist.
 Q5.4: Give evidence that [Co(NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl are ionization isomers.
Q5.4: Give evidence that [Co(NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl are ionization isomers.
Ans: When ionization isomers are dissolved in water, they ionize to give different ions. These ions then react differently with different reagents to give different products.
Page No.135 - Intext Questions
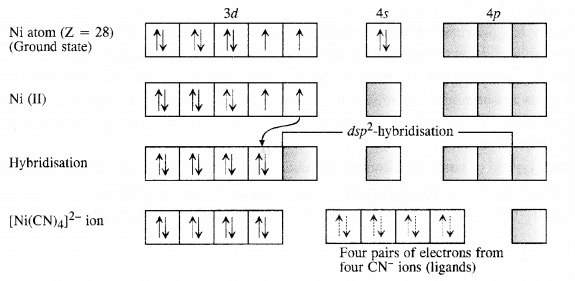
Q5.5: Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
Ans: Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2. Nickel in this complex is in + 2 oxidation state. It achieves + 2 oxidation state by the loss of the two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CN– ion is a strong field, under its attacking influence, two unpaired electrons in the 3d orbitals pair up.
 Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2 Nickel in this complex is in + 2 oxidation state. Nickel achieves + 2 oxidation state by the loss of two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CP ion is a weak field ligand, it is not in a position to cause electron pairing.
Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2 Nickel in this complex is in + 2 oxidation state. Nickel achieves + 2 oxidation state by the loss of two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CP ion is a weak field ligand, it is not in a position to cause electron pairing.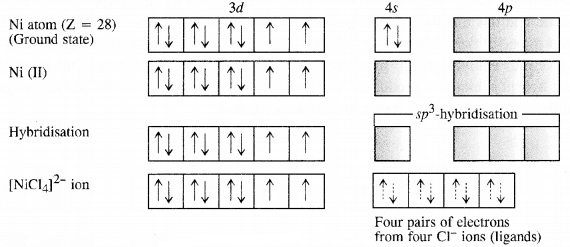 Q5.6: [NiCl4]2− is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why?
Q5.6: [NiCl4]2− is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why?Ans: In [NiCl4]2−⁻, Ni is in +2 oxidation state.
Ni (28) : 3d⁸ 4s²
Ni²⁺ : 3d⁸ 4s⁰
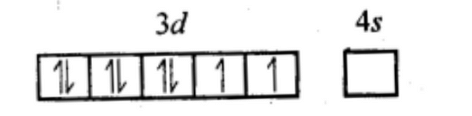
Cl⁻ is a weak field ligand. It does not pair up e⁻s. Hence, it is paramagnetic.
In [Ni(CO)4], Ni is in 0 O.S.
Ni (28) : 3d⁸ 4s²
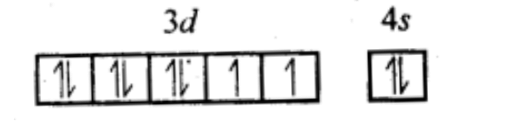
CO is a strong field ligand, as it pairs the 4s e⁻s with 3d e⁻s to give 3d¹⁰ 4s⁰. So, no unpaired e⁻ , and hence, the complex is diamagnetic.
Q5.7: [Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic. Explain.
Ans: In both [Fe(H2O)6]3+and [Fe(CN)6]3−, Fe exists in the 3 oxidation state i.e., in d5 configuration.
Since CN− is a strong field ligand, it causes the pairing of unpaired electrons. Therefore, there is only one unpaired electron left in the d-orbital.
On the other hand, H2O is a weak field ligand. Therefore, it cannot cause the pairing of electrons. This means that the number of unpaired electrons is 5.
Therefore,
Thus, it is evident that [Fe(H2O)6]3+ is strongly paramagnetic, while [Fe(CN)6]3− is weakly paramagnetic.
Q5.8: Explain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
Ans:
Q5.9: Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
Ans: In this complex, Pt is in the +2 state. It forms a square planar structure. This means that it undergoes dsp2 hybridization. Now, the electronic configuration of Pd( +2) is 5d8.
CN− being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in [Pt(CN)4]2−
Q5.10: The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacyanoion contains only one unpaired electron. Explain using Crystal Field Theory.
Ans: 
Exercises
Q5.1: Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans: The main postulates of Werner’s theory of coordination compounds are as follows:
(a)Metals possess two types of valencies: primary valency which is ionizable; and secondary valency which is non-ionizable
(b)Primary valency is satisfied by the negative ions and it is that which a metal exhibits in the formation of its simple salts.
(c)Secondary valencies are satisfied by neutral ligand or negative ligand and are those which metal exercises in the formation of its complex ions. Every cation has a fixed number of secondary valencies which are directed in space about central metal ions in certain fixed directions, e.g. In CoCl3-6NH3, valencies between Co and Cl are primary valencies, and valencies between Co and NH3 are secondary. In COCl3-6NH3, six ammonia molecules linked to Co by secondary valencies are directed to six corners of a regular octahedron and thus account for the structure of COCl3-6NH3 as follows: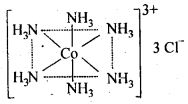 In modern theory, it is now referred as coordination number of central metal atom or ion.
In modern theory, it is now referred as coordination number of central metal atom or ion.
Q5.2: FeSO4 solution mixed with (NH4)2SO4 solution in 1:1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of Cu2+ ion. Explain why?
Ans: When FeSO4 and (NH4)2SO4 solutions are mixed in 1 : 1 molar ratio, a double salt known as Mohr’s salt is formed. It has the formula FeSO4.(NH4)2SO4.6H2O. In aqueous solution, the salt dissociates as :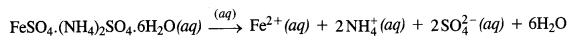 The solution gives the tests for all the ions including Fe2+ ions. On the other hand, when CuSO4 and NH3 are mixed in the molar ratio of 1 : 4 in solution, a complex [Cu(NH3)4]SO4 is formed. Since the Cu2+ ions are a part of the complex entity (enclosed in square bracket), it will not give their characteristic tests as are given by Fe2+ ions.
The solution gives the tests for all the ions including Fe2+ ions. On the other hand, when CuSO4 and NH3 are mixed in the molar ratio of 1 : 4 in solution, a complex [Cu(NH3)4]SO4 is formed. Since the Cu2+ ions are a part of the complex entity (enclosed in square bracket), it will not give their characteristic tests as are given by Fe2+ ions.
Q5.3: Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: (i) Coordination entity:
A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral molecules or negative ions ( called ligands). For example:
= cationic complex
= anionic complex
= neutral complex
(ii) Ligands
The neutral molecules or negatively charged ions that surround the metal atom in a coordination entity or a coordinal complex are known as ligands. For example, , Cl−, −OH. Ligands are usually polar in nature and possess at least one unshared pair of valence electrons.
(iii) Coordination number:
The total number of ligands (either neutral molecules or negative ions) that get attached to the central metal atom in the coordination sphere is called the coordination number of the central metal atom. It is also referred to as its ligancy.
For example:
(a) In the complex, K2[PtCl6], there as six chloride ions attached to Pt in the coordinate sphere. Therefore, the coordination number of Pt is 6.
(b) Similarly, in the complex [Ni(NH3)4]Cl2, the coordination number of the central atom (Ni) is 4.
(vi) Coordination polyhedron:
Coordination polyhedrons about the central atom can be defined as the spatial arrangement of the ligands that are directly attached to the central metal ion in the coordination sphere. For example:
(a)
(v) Homoleptic complexes:
These are those complexes in which the metal ion is bound to only one kind of a donor group. For eg: 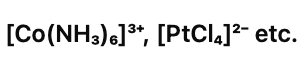
(vi) Heteroleptic complexes:
Heteroleptic complexes are those complexes where the central metal ion is bound to more than one type of a donor group.
For e.g.:
Q5.4: What is meant by unidentate, didentate & ambidentate ligands? Give two examples for each.
Ans: A ligand may contain one or more unshared pairs of electrons which are called the donor sites of ligands. Now, depending on the number of these donor sites, ligands can be classified as follows:
(a) Unidentate ligands: Ligands with only one donor site are called unidentate ligands. For e.g., NH3, Cl− etc.
(b) Didentate ligands: Ligands that have two donor sites are called didentate ligands. For e.g.,
(a) Ethane-1,2-diamine
(b) Oxalate ion
(c) Ambidentate ligands:
Ligands that can attach themselves to the central metal atom through two different atoms are called ambidentate ligands. For example:
Q5.5: Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+
(ii) [CoBr2(en)2]+
(iii) [PtCl4]2−
(iv) K3[Fe(CN)6]
(v) [Cr(NH3)3Cl3]
Ans: (i) [Co(H2O)(CN)(en)2]2+
Let the oxidation number of Co be x.
The charge on the complex is 2+.
x - 1 = +2
x = +3
(ii) [CoBr2(en)2]+
Let the oxidation number of Co be x.
The charge on the complex is +.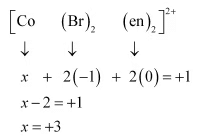 (iii) [PtCl4]2−
(iii) [PtCl4]2−
Let the oxidation number of Pt be x.
The charge on the complex is −2.
(iv) K3[Fe(CN)6]
Let the oxidation number of Fe be x.
The charge on the complex is −3.
(v) [Cr(NH3)3Cl3]
Let the oxidation number of Cr be x.
The charge on the complex is 0.
Q5.6: Using IUPAC norms write the formulas for the following: Ans:
Ans:
(a) [Zn(OH)4]2-
(b) [Pt(NH3)6]4+
(c) K2[PdCl4]
(d) [Cu(Br)4]2-
(e) [CO(NH3)6]2 (SO4)3
(f) K2[Ni(CN)4]
(g) K3 [Cr(OX)3]
(h) [CO(NH3)5ONO]2+
(i) [Pt(NH3)2Cl2]
(j) [CO(NH3)5NO2]2+
Q5.8: List various types of isomerism possible for coordination compounds, giving an example of each.
Ans: 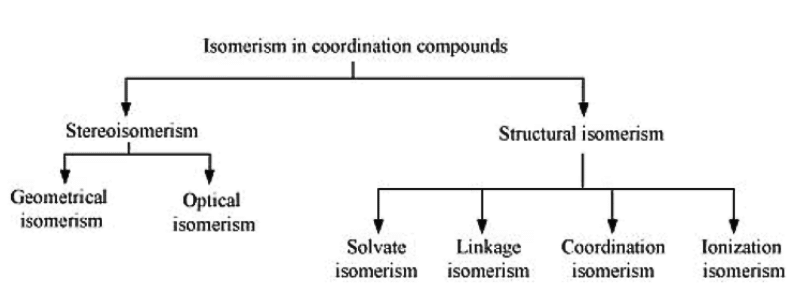
(a) Geometric isomerism:
This type of isomerism is common in heteroleptic complexes. It arises due to the different possible geometric arrangements of the ligands. For example:
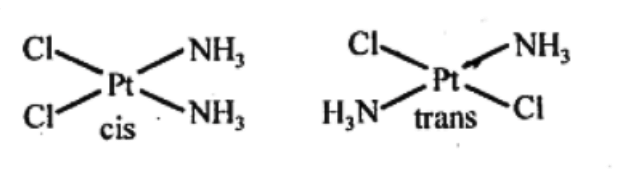
(b) Optical isomerism:
This type of isomerism arises in chiral molecules. Isomers are mirror images of each other and are non-superimposable.
(c) Linkage isomerism: This type of isomerism is found in complexes that contain ambidentate ligands. For example:
[Co(NH3)5 (NO2)]Cl2 and [Co(NH3)5 (ONO)Cl2]
Yellow form and Red form respectively.
(d) Coordination isomerism:
This type of isomerism arises when the ligands are interchanged between cationic and anionic entities of differnet metal ions present in the complex.
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]
(e) Ionization isomerism:
This type of isomerism arises when a counter ion replaces a ligand within the coordination sphere. Thus, complexes that have the same composition, but furnish different ions when dissolved in water are called ionization isomers. For e.g., Co(NH3)5SO4)Br and Co(NH3)5Br]SO4.
(f) Solvate isomerism:
Solvate isomers differ by whether or not the solvent molecule is directly bonded to the metal ion or merely present as a free solvent molecule in the crystal lattice.
[Cr[H2O)6]Cl3 [Cr(H2O)5Cl]Cl2⋅H2O [Cr(H2O)5Cl2]Cl⋅2H2O
Violet Blue-green Dark green
Q5.9: How many geometrical isomers are possible in the following coordination entities?
(i) [Cr(C2O4)3]3−
(ii) [Co(NH3)3Cl3]
Ans:
(i) For [Cr(C2O4)3]3−, no geometric isomer is possible as it is a bidentate ligand.
(ii) [Co(NH3)3Cl3]
Two geometrical isomers are possible.
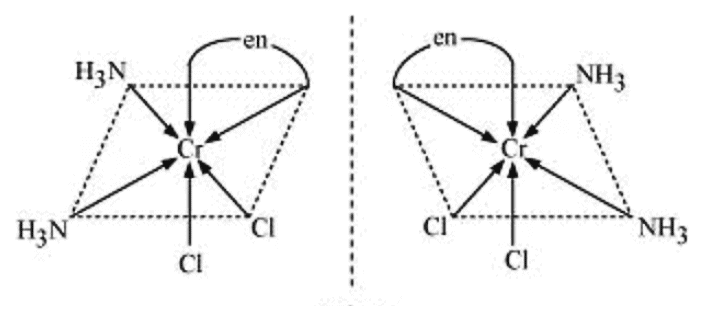
Q5.10: Draw the structures of optical isomers of:
(i) [Cr(C2O4)3]3−
(ii) [PtCl2(en)2]2+
(iii) [Cr(NH3)2Cl2(en)]+
Ans: (i) [Cr(C2O4)3]3−
(ii) [PtCl2(en)2]2+
(iii) [Cr(NH3)2Cl2(en)]+
Q5.11: Draw all the isomers (geometrical and optical) of:
(i) [CoCl2(en)2] +
(ii) [Co(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl2(en)]+
Ans:
(i) [CoCl2(en)2]+
Geometrical isomerism
Optical isomerism
Since only cis isomer is optically active, it shows optical isomerism.
In total, three isomers are possible.
(ii) [Co(NH3)Cl(en)2]2+
Geometrical isomerism
Optical isomerism
Since only cis isomer is optically active, it shows optical isomerism.
(iii) [Co(NH3)2Cl2(en)]
Q5.12: Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
Ans: [Pt(NH3)(Br)(Cl)(py)]
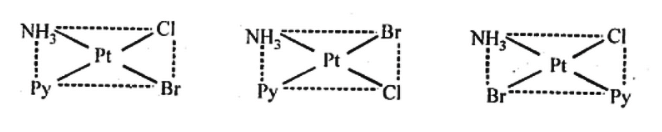
From the above isomers, none will exhibit optical isomers. Square planner complexes rarely show optical isomerization. They do so only in the presence of unsymmetrical chelating agents.
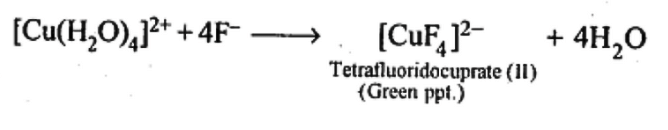
Q5.13: Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride, and
(ii) a bright green solution with aqueous potassium chloride
Explain these experimental results.
Ans: Aqueous CuSO4 exists as [Cu(H2O)4]SO4. It is blue in colour due to the presence of [Cu[H2O)4]2 ions.
(i) When KF is added: the weak H20 ligands are replaced by F– ligands forming [CUF4]2- ions which is a green precipitate.
 (ii) When KCl is added: Cl– ligands replace the weak H20 ligands forming [CuCl4]2- ion which has bright green colour.
(ii) When KCl is added: Cl– ligands replace the weak H20 ligands forming [CuCl4]2- ion which has bright green colour.
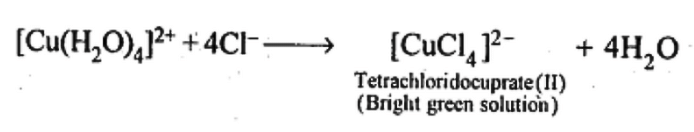
In both these cases, the weak field ligand water is replaced by the F− and Cl− ions.
Q5.14: What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
Ans:
First cupric cyanide is formed which decomposes to give cuprous cyanide and cyanogen gas. Cuprous cyanide dissolves in excess of potassium cyanide to form the complex, K3[Cu(CN)4],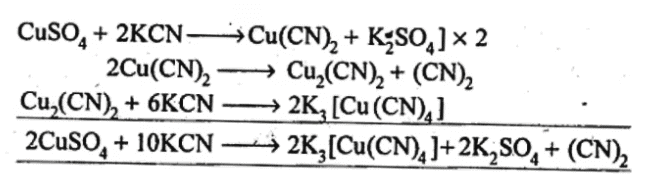 Thus, coordination entity formed in the above reaction is [Cu(CN)4]3-. As CN– is a strong ligand, the complex ion is highly stable and does not dissociate/ionize to give Cu2+ ions. Hence, no precipitate,with H2S is formed.
Thus, coordination entity formed in the above reaction is [Cu(CN)4]3-. As CN– is a strong ligand, the complex ion is highly stable and does not dissociate/ionize to give Cu2+ ions. Hence, no precipitate,with H2S is formed.
Q5.15: Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4−
(ii) [FeF6]3−
(iii) [Co(C2O4)3]3−
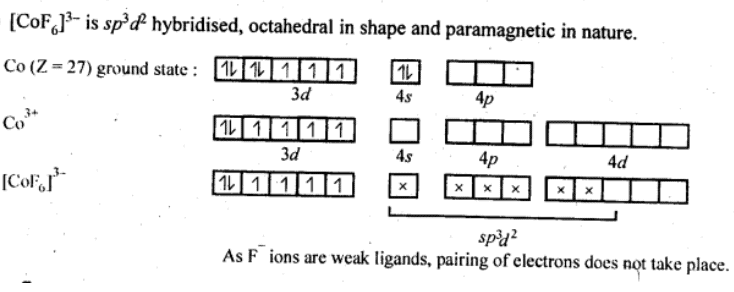
(iv) [CoF6]3−
Ans: (i) [Fe(CN)6]4−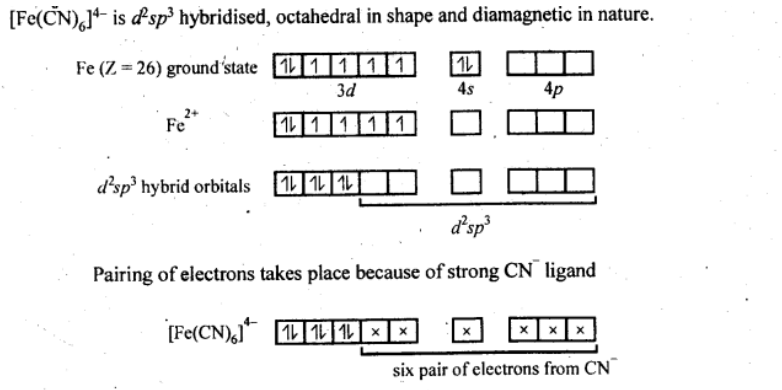
(ii) [FeF6]3−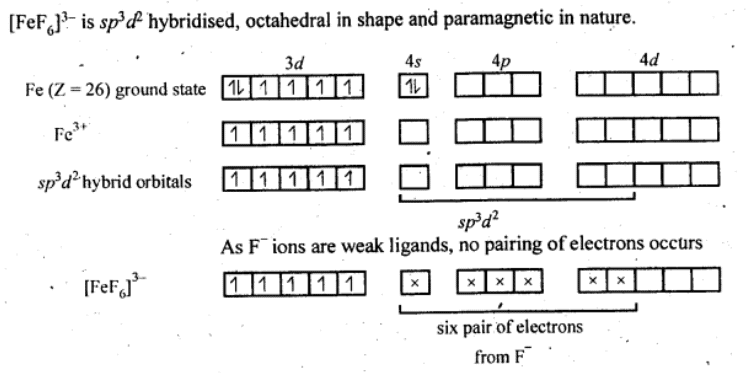
(iii) [Co(C2O4)3]3−
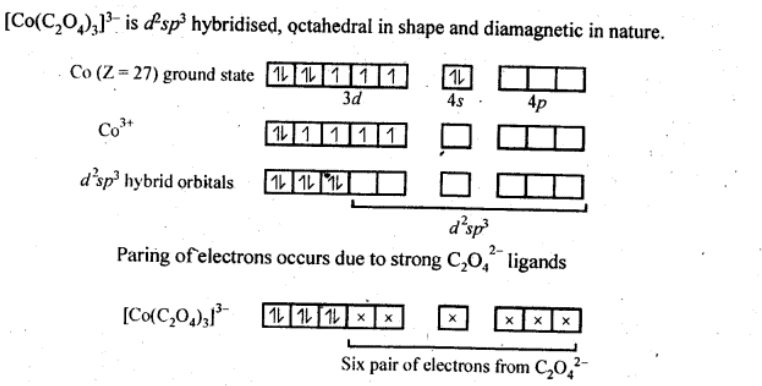
(iv) [CoF6]3−
 Q5.16: Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Q5.16: Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Ans:
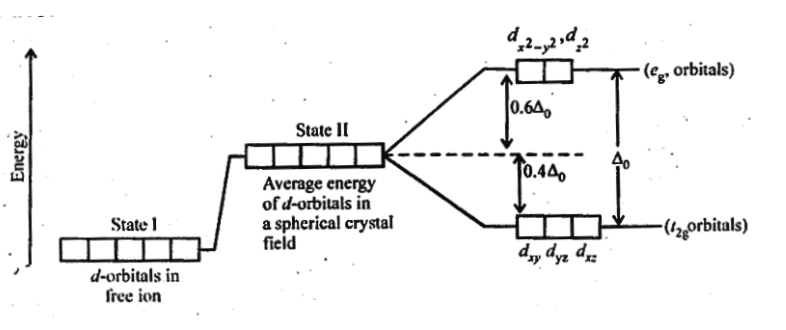
The splitting of the d orbitals in an octahedral field takes place in such a way that , experience a rise in energy and form the eg level, while dxy, dyz and dzx experience a fall in energy and form the t2g level.
Q5.17: What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans: A spectrochemical series is the arrangement of common ligands in the increasing order of their crystal-field splitting energy (CFSE) values. The ligands present on the R.H.S of the series are strong field ligands while that on the L.H.S are weak field ligands. Also, strong field ligands cause higher splitting in the d orbitals than weak field ligands.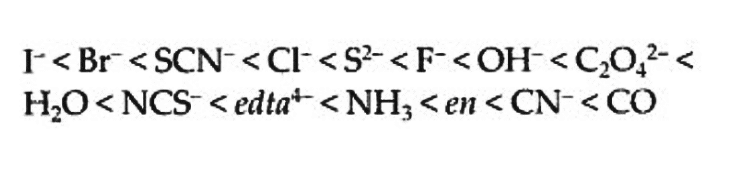 Q5.18: What is crystal field splitting energy? How does the magnitude of Δo decide the actual configuration of d-orbitals in a coordination entity?
Q5.18: What is crystal field splitting energy? How does the magnitude of Δo decide the actual configuration of d-orbitals in a coordination entity?
Ans: The degenerate d-orbitals (in a spherical field environment) split into two levels i.e., eg and t2g in the presence of ligands. The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels (eg and t2g) is called the crystal-field splitting energy. It is denoted by Δo.
When the ligands approach a transition metal ion, the d-orbitals split into two sets, one with lower energy and the other with higher energy. The difference of energy between the two sets of orbitals is called crystal field splitting energy (Δ0 for octahedral field). If Δ0 < P (pairing energy), the fourth electron enters
one of the e°g, orbitals giving the configuration t32ge1g, thus forming high spin complexes. Such ligands for which Δ0 < P are called weak field ligands. If Δ0 > P, the fourth electron pairs up in one of the t2g orbitals giving the configuration t42ge1g thereby forming low spin complexes. Such ligands for which Δ0> P are called strong field ligands.
Q5.19: [Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Ans: 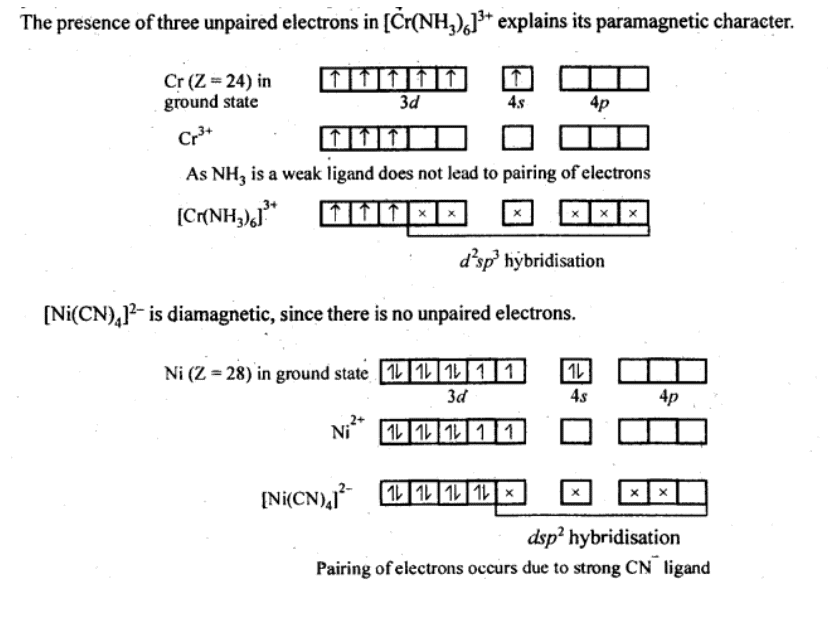 Q5.20: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2− is colourless. Explain.
Q5.20: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2− is colourless. Explain.
Ans: In [Ni(H20)6]2+, Ni is in a + 2 oxidation state and has 3d8 electronic configuration, in which two unpaired electrons do not pair in the presence of the weak H2O ligand. Hence, it is coloured. The d-d transition absorbs red light and the complementary light emitted is green.
In [Ni(CN)4]2- Ni is also in a + 2 oxidation state and has 3d8 electronic configuration. But in the presence of strong ligand CN– the two unpaired electrons in the 3d orbitals pair up. Thus, there is no unpaired electron present. Hence, it is colourless.
Q5.21: [Fe(CN)6]4− and [Fe(H2O)6]2+ are of different colours in dilute solutions. Why?
Ans: In both complexes, Fe is in a + 2 oxidation state with a d6 configuration. This means that it has four unpaired electrons. Both CN– ion and H2O molecules which act as ligands occupy different relative positions in the spectrochemical series. They differ in crystal field splitting energy (∆0). Quite obviously, they absorb radiations corresponding to different wavelengths/frequencies from the visible region of light. (VIBGYOR) and the transmitted colours are also different. This means that the complexes have different colours in solutions.
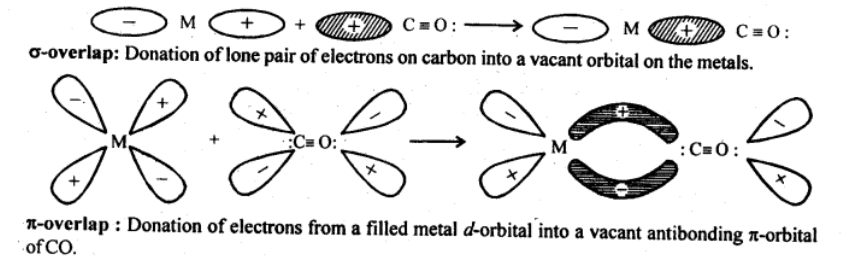
Q5.22: Discuss the nature of bonding in metal carbonyls.
Ans: In metal carbonyl, the metal-carbon bond (M – C) possess both the σ and π -bond characters. The bond is formed by the overlap of the atomic orbital of metal with that of the C-atom of carbon monoxide in the following sequence:
(a) σ -bond is first formed between metal and carbon when a vacant d-orbital of a metal atom overlaps with an orbital containing a lone pair of electrons on the C-atom of carbon monoxide (: C = O:)
(b) In addition to σ -bond in metal carbonyl, the electrons from filled d-orbitals of a transition metal atom/ ion are back donated into antibonding π-orbitals of carbon monoxide. This stabilizes the metal-ligand bonding. The above two concepts are shown in the following figure:
 Q5.23: Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes:
Q5.23: Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes:
(i) K3[Co(C2O4)3]
(ii) cis-[Cr(en)2Cl2]Cl
(iii) (NH4)2[CoF4]
(iv) [Mn(H2O)6]SO4
Ans: (i) K3[Co(C2O4)3]
The central metal ion is Co.
Its coordination number is 6.
The oxidation state can be given as:
x + (−2)3 = −3
x = 3
The d orbital occupation for Co3 is t2g6eg0.
(ii) cis-[Cr(en)2Cl2]Cl
The central metal ion is Cr.
The coordination number is 6.
The oxidation state can be given as:
x + 2(0) + 2(−1) = 1
x − 2 = 1
x = 3
The d orbital occupation for Cr3 is t2g3eg0.
(iii) (NH4)2[CoF4]
The central metal ion is Co.
The coordination number is 4.
The oxidation state can be given as:
x − 4 = −2
x = 2
The d orbital occupation for Co2 is eg4 t2g3.
(iv) [Mn(H2O)6]SO4
The central metal ion is Mn.
The coordination number is 6.
The oxidation state can be given as:
x + 0 = +2
x = +2
The d orbital occupation for Mn is t2g3 eg2.
Q5.24: Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also, give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O
(ii) [Co(NH3)5Cl]Cl2
(iii) CrCl3(py)3
(iv) Cs[FeCl4]
(v) K4[Mn(CN)6]
Ans: (i) Potassium diaquadioxalatochromate (III) trihydrate.
Oxidation state of chromium = x + 0 + 2 (-2) = – 1
x = + 3
Electronic configuration: 3d3= t32ge0g
Coordination number = 6
Shape: octahedral
Stereochemistry:
∼ 4BM
(ii) [Co(NH3)5Cl]Cl2
IUPAC name: Pentaamminechloridocobalt(III) chloride
The oxidation state of Co = x + 0 -1 = + 2
x = + 3
Coordination number = 6
Shape: octahedral.
Electronic configuration: d6: t2g6e°g .
Stereochemistry:
Magnetic Moment = 0
(iii) CrCl3(py)3
IUPAC name: Trichloridotripyridinechromium (III)
The oxidation state of chromium = x - 3 + 0 = 0
x= +3
Electronic configuration for d3 = t2g3e°g
Coordination number = 6
Shape: octahedral.
Stereochemistry:
Both isomers are optically active. Therefore, a total of 4 isomers exist.
∼ 4BM
(iv) Cs[FeCl4]
IUPAC name: Caesium tetrachloroferrate (III)
The oxidation state of Fe = x - 4 = -1
x = +3
Electronic configuration of d6 = t2g3 eg2
Coordination number = 4
Shape: tetrahedral
Stereochemistry: optically inactive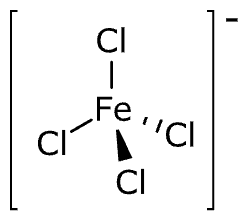 Magnetic moment:
Magnetic moment:
(v) K4[Mn(CN)6]
Potassium hexacyanomanganate(II)
The oxidation state of manganese =x - 6 = - 4
x = +2
Electronic configuration: d5 : t2g5
Coordination number = 6
Shape: octahedral.
Streochemistry: optically inactive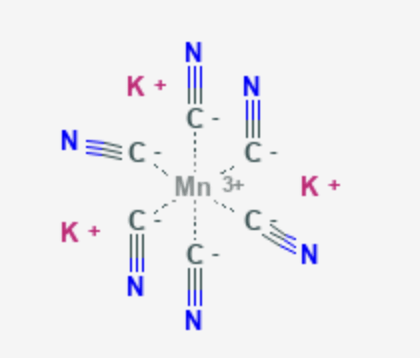
Q5.25: Explain the purple colour of the complex, [Ti(H2O)6]3+ with the help of crystal field theory.
Ans: In the complex, the Ti atom has an oxidation state of +3. Its outer electronic configuration is 3d1 It has one unpaired electron. This unpaired electron is excited from the t2g level to eg level by absorbing yellow light and hence appears violet-coloured.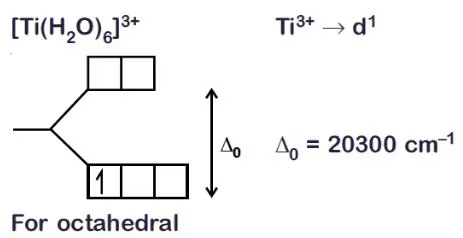 Q5.26: What is meant by the chelate effect? Give an example.
Q5.26: What is meant by the chelate effect? Give an example.
Ans: When a bidentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metal ion, a five or six-membered ring is formed, the effect is called the chelate effect. We can say that complexes containing chelate rings are more stable than complexes without rings.
For example: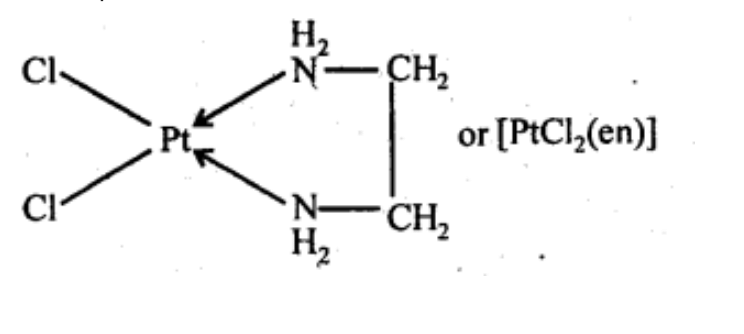 Q5.27: Discuss briefly giving an example in each case the role of coordination compounds in:
Q5.27: Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological system
(ii) medicinal chemistry
(iii) analytical chemistry
(iv) extraction/metallurgy of metals
Ans: (i) Role of coordination compounds in biological systems:
Coordination compounds are of great importance in biological systems. The pigment responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin, the red pigment of blood that acts as an oxygen carrier is a coordination compound of iron. Vitamin B12, cyanocobalamine, the anti-pernicious anemia factor, is a coordination compound of cobalt. Among the other compounds of biological importance with coordinated metal ions are enzymes like carboxypeptidase A and carbonic anhydrase (catalysts of biological systems).
(ii) Role of coordination compounds in medicinal chemistry:
There is growing Interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant/animal systems. Thus, excess copper and iron are removed by the chelating ligands D-penicillamine and desferrioxime B via the formation of coordination compounds.
EDTA is used in the treatment of lead poisoning. Some coordination compounds of platinum effectively inhibit the growth of tumours. Examples are: ds-platin and related compounds.
(iii) Role of coordination compounds in analytical chemistry:
Coordination compounds find use in many qualitative and quantitative chemical analyses. The familiar color reactions given by metal ions with a number of ligands (especially chelating ligands), as a result of the formation of coordination entities, form the basis for their detection and estimation by classical and instrumental methods of analysis. Examples of such reagents include EDTA, DMG (dimethylglyoxime), α–nitroso–β–naphthol, cupron, etc.
(iii) Role of coordination compounds in extraction or metallurgy of metals:
Some important extraction processes of metals, like those of silver and gold, make use of complex formation. Gold, for example, combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN)2]- in aqueous solution. Gold can be separated in metallic form from this solution by the addition of zinc.
Q5.28: How many ions are produced from the complex Co(NH3)6]Cl2 in solution?
(i) 6
(ii) 4
(iii) 3
(iv) 2
Ans: (iii) The given complex can be written as [Co(NH3)6]Cl2.
Thus, [Co(NH3)6]+ along with two Cl− ions are produced.
Q5.29: Amongst the following ions which one has the highest magnetic moment value?
(i) [Cr(H2O)6]3+
(ii) [Fe(H2O)6]2+
(iii) [Zn(H2O)6]2+
Ans: (i) No. of unpaired electrons in [Cr(H2O)6]3+ = 3
~ 4BM
(ii) No. of unpaired electrons in [Fe(H2O)6]2+ = 4
(iii) No. of unpaired electrons in [Zn(H2O)6]2+ = 0
Hence, [Fe(H2O)6]2+ has the highest magnetic moment value.
Q5.30: Amongst the following, the most stable complex is
(i) [Fe(H2O)6]3+
(ii) [Fe(NH3)6]3+
(iii) [Fe(C2O4)3]3−
(iv) [FeCl6]3−
Ans: We know that the stability of a complex increases by chelation. Therefore, the most stable complex is [Fe(C2O4)3]3−.
Q5.31: What will be the correct order for the wavelengths of absorption in the visible region for the following:
[Ni(NO2)6]4−, [Ni(NH3)6]2+ , [Ni(H2O)6]2+
Ans: The central metal ion in all three complexes is the same. Therefore, absorption in the visible region depends on the ligands. The order in which the CFSE values of the ligands increase in the spectrochemical series is as follows:
H2O < NH3 < NO2−
Thus, the amount of crystal-field splitting observed will be in the following order:

Hence, the wavelengths of absorption in the visible region will be in the order:
[Ni(H2O)6]2+ > [Ni(NH3)6]2+ > [Ni(NO2)6]4−
The document NCERT Solutions Class 12 Chemistry Chapter 5 - Coordination Compounds is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|278 docs|78 tests
|
FAQs on NCERT Solutions Class 12 Chemistry Chapter 5 - Coordination Compounds
| 1. What are coordination compounds? |  |
Ans. Coordination compounds are molecules or ions that have a central metal atom or ion bonded to a surrounding array of molecules or ions, known as ligands.
| 2. How do ligands coordinate with metal ions in coordination compounds? |  |
Ans. Ligands coordinate with metal ions in coordination compounds by donating a pair of electrons to the metal ion, forming coordinate covalent bonds.
| 3. What is the coordination number of a metal ion in a coordination compound? |  |
Ans. The coordination number of a metal ion in a coordination compound is the number of coordinate covalent bonds formed between the metal ion and the ligands.
| 4. What is the difference between a coordination compound and a complex ion? |  |
Ans. A coordination compound consists of a central metal atom or ion bonded to ligands, while a complex ion is a charged species consisting of a central metal ion bonded to surrounding ligands.
| 5. How are coordination compounds named? |  |
Ans. Coordination compounds are named by listing the ligands first in alphabetical order, followed by the central metal ion with a Roman numeral to indicate its charge, if necessary.
Related Searches

















