NCERT Solutions Class 12 Chemistry Chapter 15 - Polymers
Ques 15.1: What are polymers?
Ans:- Polymers are high molecular mass macromolecules, which consist of repeating structural units derived from monomers. Polymers have a high molecular mass (103 − 107 u). In a polymer, various monomer units are joined by strong covalent bonds. These polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6, 6 are examples of polymers.
Ques 15.2: How are polymers classified on the basis of structure?
Ans:- Polymers are classified on the basis of structure as follows:
1. Linear polymers:
These polymers are formed of long straight chains. They can be depicted as:

For e.g., high density polythene (HDP), polyvinyl chloride, etc.
2. Branched chain polymers:
These polymers are basically linear chain polymers with some branches.These polymers are represented as:

For e.g., low density polythene (LDP), amylopectin, etc.
3. Cross-linked or Network polymers:
These polymers have many cross-linking bonds that give rise to a network-like structure. These polymers contain bi-functional and tri-functional monomers and strong covalent bonds between various linear polymer chains. Examples of such polymers include bakelite and melmac.

Ques 15.3: Write the names of monomers of the following polymers:



Ans:- (i) Hexamethylenediamine  and adipic acid
and adipic acid 
(ii)

(iii) Tetrafluoroethene (CF2 = CF2)
Ques 15.4: Classify the following as addition and condensation polymers: Terylene, Bakelite, Polyvinyl chloride, Polythene.
Ans:- Addition polymers:
Polyvinyl chloride, polythene
Condensation polymers:
Terylene, bakelite
Ques 15.5: Explain the difference between Buna-N and Buna-S.
Ans:- Buna − N is a copolymer of 1, 3−butadiene and acrylonitrile.
Buna − S is a copolymer of 1, 3−butadiene and styrene.
Ques 15.6: Arrange the following polymers in increasing order of their intermolecular forces.
(i) Nylon 6, 6, Buna-S, Polythene.
(ii) Nylon 6, Neoprene, Polyvinyl chloride.
Ans:- Different types of polymers have different intermolecular forces of attraction. Elastomers or rubbers have the weakest while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate intermolecular forces of attraction. Hence, the increasing order of the intermolecular forces of the given polymers is as follows:
(i) Buna − S < polythene < Nylon 6, 6
(ii) Neoprene < polyvinyl chloride < Nylon 6
Ques 15.7: Explain the terms polymer and monomer.
Ans:- Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (103 − 107 u). In a polymer, various monomer units are joined by strong covalent bonds. Polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6, 6 are examples of polymers.
Monomers are simple, reactive molecules that combine with each other in large numbers through covalent bonds to give rise to polymers. For example, ethene, propene, styrene, vinyl chloride.
Ques 15.8: What are natural and synthetic polymers? Give two examples of each type.
Ans:- Natural polymers are polymers that are found in nature. They are formed by plants and animals. Examples include protein, cellulose, starch, etc.
Synthetic polymers are polymers made by human beings. Examples include plastic (polythene), synthetic fibres (nylon 6, 6), synthetic rubbers (Buna − S).
Ques 15.9: Distinguish between the terms homopolymer and copolymer and give an example of each.
Ans:-
Homopolymer | Copolymer |
The polymers that are formed by the polymerization of a single monomer are known as homopolymers. In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethene. | The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna−S is a copolymer of 1, 3-butadiene and styrene. |
Ques 15.10: How do you explain the functionality of a monomer?
Ans:- The functionality of a monomer is the number of binding sites that is/are present in that monomer.
For example, the functionality of monomers such as ethene and propene is one and that of 1, 3-butadiene and adipic acid is two.
Ques 15.11: Define the term polymerisation.
Ans:- Polymerization is the process of forming high molecular mass (103 − 107 u) macromolecules, which consist of repeating structural units derived from monomers. In a polymer, various monomer units are joined by strong covalent bonds.
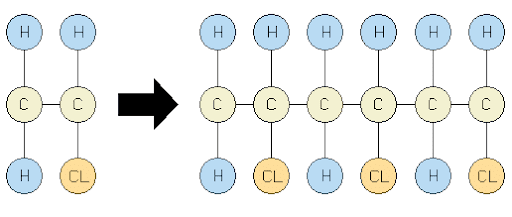 Polymerisation
Polymerisation
Ques 15.12: Is  , a homopolymer or copolymer?
, a homopolymer or copolymer?
Ans:-  is a homopolymer because it is obtained from a single monomer unit, NH2−CHR−COOH.
is a homopolymer because it is obtained from a single monomer unit, NH2−CHR−COOH.
Ques 15.13: In which classes, the polymers are classified on the basis of molecular forces?
Ans:- On the basis of magnitude of intermolecular forces present in polymers, they are classified into the following groups:
(i) Elastomers
(ii) Fibres
(iii) Thermoplastic polymers
(iv) Thermosetting polymers
Ques 15.14: How can you differentiate between addition and condensation polymerisation?
Ans:- Addition polymerization is the process of repeated addition of monomers, possessing double or triple bonds to form polymers. For example, polythene is formed by addition polymerization of ethene.

Condensation polymerization is the process of formation of polymers by repeated condensation reactions between two different bi-functional or tri-functional monomers. A small molecule such as water or hydrochloric acid is eliminated in each condensation. For example, nylon 6, 6 is formed by condensation polymerization of hexamethylenediamine and adipic acid.

Ques 15.15: Explain the term copolymerisation and give two examples.
Ans:- The process of forming polymers from two or more different monomeric units is called copolymerization. Multiple units of each monomer are present in a copolymer. The process of forming polymer Buna−S from 1, 3-butadiene and styrene is an example of copolymerization.

Nylon 6, 6 is also a copolymer formed by hexamethylenediamine and adipic acid.

Ques 15.16: Write the free radical mechanism for the polymerisation of ethene.
Ans:- Polymerization of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyal peroxide as the initiator.
The reaction involved in this process is given below:

Chain Propagating Step



Ques 15.17: Define thermoplastics and thermosetting polymers with two examples of each.
Ans:- Thermoplastic polymers are linear (slightly branched) long chain polymers, which can be repeatedly softened and hardened on heating. Hence, they can be modified again and again. Examples include polythene, polystyrene.
Thermosetting polymers are cross-linked or heavily branched polymers which get hardened during the moulding process. These plastics cannot be softened again on heating. Examples of thermosetting plastics include bakelite, urea-formaldehyde resins.
Ques 15.18: Write the monomers used for getting the following polymers.
(i) Polyvinyl chloride
(ii) Teflon
(iii) Bakelite
Ans:- (i) Vinyl chloride (CH2=CHCl)
(ii) Tetrafluoroethylene (CF2 = CF2)
(iii) Formaldehyde (HCHO) and phenol (C6H5OH)
Ques 15.19: Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Ans:- One common initiator used in free radical addition polymerization is benzoyl peroxide. Its structure is given below.

Ques 15.20: How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Ans:- Natural rubber is a linear cis-polyisoprene in which the double bonds are present between C2 and C3 of the isoprene units.

Because of this cis-configuration, intermolecular interactions between the various strands of isoprene are quite weak. As a result, various strands in natural rubber are arranged randomly. Hence, it shows elasticity.
Ques 15.21: Discuss the main purpose of vulcanisation of rubber.
Ans:- Natural rubber though useful has some problems associated with its use. These limitations are discussed below:
1. Natural rubber is quite soft and sticky at room temperature. At elevated temperatures (> 335 K), it becomes even softer. At low temperatures (< 283 K), it becomes brittle. Thus, to maintain its elasticity, natural rubber is generally used in the temperature range of 283 K-335 K.
2. It has the capacity to absorb large amounts of water.
3. It has low tensile strength and low resistance to abrasion.
4. It is soluble in non-polar solvents.
5. It is easily attacked by oxidizing agents.
Vulcanization of natural rubber is done to improve upon all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between 373 K and 415 K.
Ques 15.22: What are the monomeric repeating units of Nylon-6 and Nylon-6, 6?
Ans:- The monomeric repeating unit of nylon 6 is ,  which is derived from Caprolactam.
which is derived from Caprolactam.
The monomeric repeating unit of nylon 6, 6 is ,  which is derived from hexamethylene diamine and adipic acid.
which is derived from hexamethylene diamine and adipic acid.
Ques 15.23: Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Buna-N
(iii) Dacron
(iv) Neoprene
Ans:-

Ques 15.24: Identify the monomer in the following polymeric structures.
(i)

(ii)

Ans:- (i) The monomers of the given polymeric structure are decanoic acid  and hexamethylene diamine
and hexamethylene diamine 
(ii) The monomers of the given polymeric structure are

Ques 15.25: How is dacron obtained from ethylene glycol and terephthalic acid?
Ans:- The condensation polymerisation of ethylene glycol and terephthalic acid leads to the formation of dacron.

Ques 15.26: What is a biodegradable polymer? Give an example of a biodegradable aliphatic polyester.
Ans:- A polymer that can be decomposed by bacteria is called a biodegradable polymer.
Poly-β-hydroxybutyrate-CO-β- hydroxyvalerate (PHBV) is a biodegradable aliphatic polyester.

|
916 docs|393 tests
|
FAQs on NCERT Solutions Class 12 Chemistry Chapter 15 - Polymers
| 1. What are polymers and why are they important? |  |
| 2. What is the difference between natural and synthetic polymers? |  |
| 3. How are polymers classified based on their structure? |  |
| 4. What is polymerization and what are the different types of polymerization reactions? |  |
| 5. How can polymers be recycled? |  |
|
916 docs|393 tests
|

|
Explore Courses for UPSC exam
|

|


















