Carboxylic Acids & Acids Derivatives, Class 12, Chemistry Detailed Chapter Notes PDF Download
Nomenclature of Aldehydes and Ketones - Aldehydes, Ketones and Carboxylic Acids, Class 12, Chemistry
Introduction :
Table – 1 : IUPAC Nomenclature of Acid derivatives :-
Dicarboxylic Acids
If the subsituent is a second carboxyl gropu, we have a dicarboxylic acid. For example :
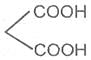
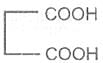
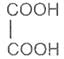
Oxalic acid Malonic acid -1, 3- Succinic acid -1, 4-
Ethanedioic acid dioicacid dioic acid
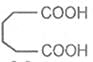
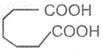
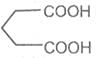
Glutaric acid Adipic acid Pimelic acid
Pentane-1, 5-dioicacid Hexane-1, 6-dioicacid Heptane-1, 7- dioci acid
HOOCCH2COOH HOOCCH2CH2COOH HOOCCH2CH2CH2CH2COOH
Malonic acid Succinic acid Adipic acid
Propanedioic acid Butanedioic acid Hexanedioic acid
========================================================
Physical Properties of Aldehydes and Ketones - Aldehydes, Ketones and Carboxylic Acids, Class 11, Chemistry
Physical properties of acids and acid derivatives :
1 Boiling point :
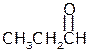
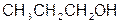
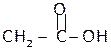 Acetic acid, 1-propanol Propionaldehyde
Acetic acid, 1-propanol Propionaldehyde
bp 1180C bp910C bp490C
The high boiling points of carboxylic acids is the result of formation of a stable hydrogen-bonded dimer.
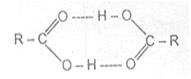
Hydrogen bonded acid dimer
2 Solubility :
Carboxylic acids form hydrogen bonds with water and the lower molecular –weight carboxylic acids (upto 4 carbon atoms) are miscible with water.
Acid derivatives (esters, acid chlorides, anhydride, nitriles and amides) are soluble organic solvents such as alcohols, ethers, chlorinated alkanes and aromatic hydrocarbons.
Methods of preparation of carboxylic acids
1. Synthese of carboxylic acids by the carboxylation of grignard reagents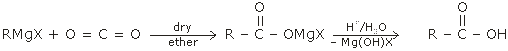
2- Synthesis of Carboxylic acids by the hydrolysis of nitriles Mechanism :

Nitrile carboxylic acid
Chemical Reactions
Reaction of carbroxylic acid with aqueous sodium carbonatge solution produces brisk efferuescnce. However most phenols do not produce effervescence. Therefore, the reaction may be used to distinguish between carboxylic acids and phenols.
If we follow the forward in this mechanism, we have the mechanism for the acid catalysed esterification fo an acid. If however, we follow the reverse reactions, we have the meachanism for the acid catalysed hydrolysis of an ester. Acid catalysed ester hydrolysis.
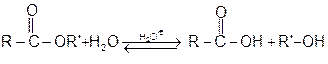
3 Formation of amides :
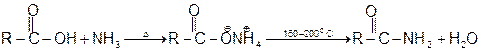
4 Formation of acid anhydride :
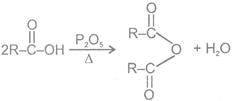
4- Decarboxylation reactions:
1 Soda-lime decarboxylation :
General reaction:
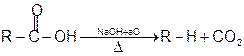
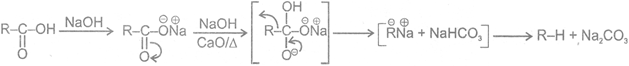
2 Decarboxylation of b- keto carboxylic acids :
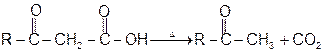
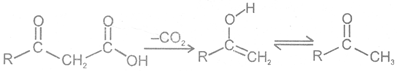
 - keto acid
- keto acid
3 Kolbe electrolysis
4 Hunsdiecker Reaction (Brome-decarboxylation):
5. HVZ Reaction (Halogenation of aliphatic acids and Substituted acids)
Carboxylic Acid Derivatives
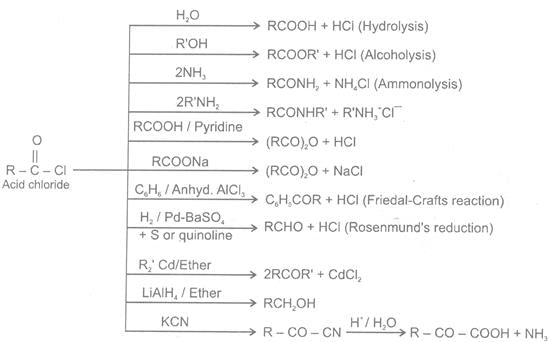
Closely related to the carboxylic acids and to each other are a number of chemical families known as functional derivatives of carboxylic acids : acid chloride, anhydrides, amide, and esters, These derivatives are compounds in which the -OH of a carboxyl group has been replaced by–Cl, -OOCR, -NR2 or –OR
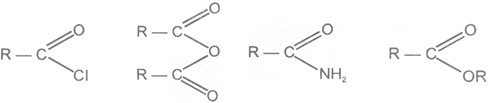
Acid chloride Anhydride Amide Ester
They all contain the acyl group ,
(A) Acid halides
Methods of preparations of Acyl halides
(i) RCOOH PCl5 → RCOCl POCl3 HCl
(ii) 3RCOOH PCl3 → 3RCOCl H3PO3
(iii) RCOOH SOCl2 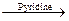 RCOCl SO2 HCl
RCOCl SO2 HCl
Ex. 3CH3 COONa PCl3 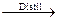 3CH3 COCl Na3PO3
3CH3 COCl Na3PO3
Acetyl chloride
Ex. 2C6H5COONa POCl3 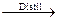 2C6H5COCl NaCl NaPO3
2C6H5COCl NaCl NaPO3
Sod. benzoate Benzoyl chloride
Chemical Reactions
1 Reaction with carboxylic acids
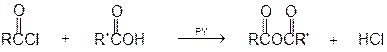
Acyl Carboxylic Acid Hydrogen
chloride acid anhydride chloride
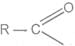
2 Reaction with alcohols
Acyl chlorides react with alcohols to form esters. The reaction is typically carried out in the presence of pyridine.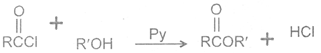
Acyl Alcohol Ester Hydrogen
choride chloride

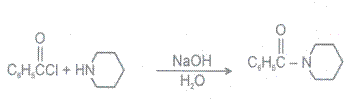
3 Hydrolysis


4 Reaction of acid halide with organometallic
(a) with Grignard reagent
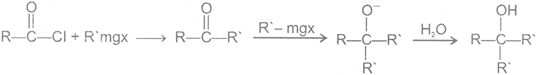
(b) Reaction with Gilmann reagent

5 Reduction of acid halided
(a) Reduction LiAlH4
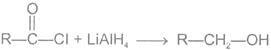
(b) Reduction with H2/Pd/BaSO4 Rosenmund reduction
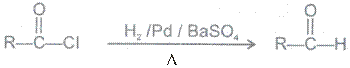
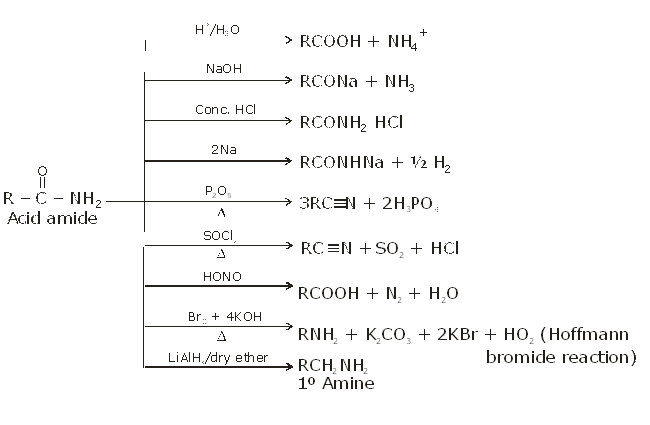
(B) Acid amides
Methods of preparation of acids amides
1. By reaction of esters with ammonia and amines

Ammonia is more nucleophilic than water, making it possible to carry out this reaction using aqueous ammonia.
Ex.
2. From acid halides
RCOCl 2NH3 → RCONH2 NH4Cl
3. From anhydride
(RCO)2O 2NH3 → RCONH2 RCOO NH4
4. From esters
RCOOR NH3 → RCONH2 R’OH
5. From ammonium salt of carboxylic acid
RCOONH4  RCONH2 H2O
RCONH2 H2O
CH3 COONH4 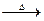 CH3CONH2
CH3CONH2
6. From cyanides
R – C º N H2O 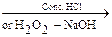 R – CONH2
R – CONH2
CH3C = N H2O 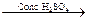 CH3 - CONH2
CH3 - CONH2
7.
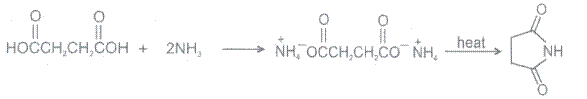
Chemical Reactions
(1) Hoffmann rearrangement
General reaction

(2) Hydrolysis of amides

In acid, however, the amine is protonated, giving an ammonium ion, R2’ 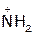

Summary of reaction of amide:
(C) Esters
Methods of Preparation
(i) CH3 COOH C2H5OH 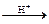 CH3COOC2 H5 H2O
CH3COOC2 H5 H2O
Acetic acid
C6H5COOH CH3OH 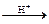 C6H5 COOCH3 H2O
C6H5 COOCH3 H2O
(ii) CH3 COCl C2H5OH 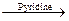 CH3COOC2H5 HCl
CH3COOC2H5 HCl
Alcohols react with acyl chlorides by nucleophilic acyl substitution to yield esters. These reactions are typically perormed in the presence of a weak base such as pyridine.

Summary of reaction of esters :

(D) Acid anhydrides
Methods of Preparation of acid anhydrides
1- From carboxylic acids
Ex. CH3COOH HOOCCH3  CH3CO.O.COCH3 H2O
CH3CO.O.COCH3 H2O
Acetic acid Acetic anhydride
Ex.
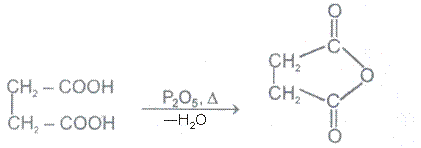
Ex.
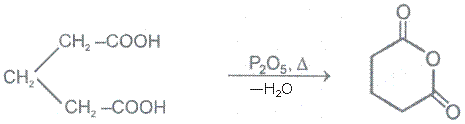
2. From acid and acid halide
Ex. CH3COOH CH3COCl 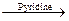 CH3 CO.O.COCH3 HCl
CH3 CO.O.COCH3 HCl
Ex. CH3COCl CH3COONa  CH3CO.O.COCH3 NaCl
CH3CO.O.COCH3 NaCl
Chemical Reactions
1 Reaction with aromatic compounds (Friedel crafts acylation)

2 Reaction with alcohols
Ex.

3 Reaction with ammonia and amines

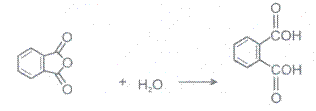
4 Hydrolysis
Acid anhydrides react with to yield two carboxylic. Cycylic anhydides yield dicarboxylic acids.
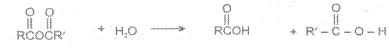
5- Heating Effects:
a Heating effect on monocarboxylic acid
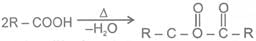
b Heating effect on dicarboxylic acid
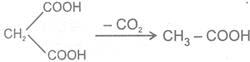
c Heating effect on Hydroxy acids
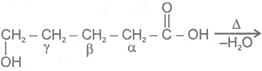
1- 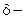 Hydroxy acid
Hydroxy acid
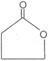
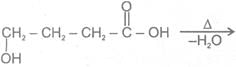
2.  Hyroxy acid
Hyroxy acid
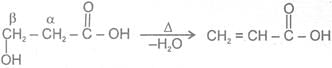
3- 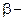 Hydroxy acid
Hydroxy acid
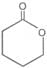
Since 4 or 8 membered rings are less stable the refore b-Hydroxy acids on heating produce a, b unsaturated carboxylic acid.
4- aa -Hyroxy acid
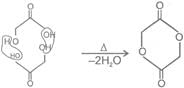
Û Heating effect on esters
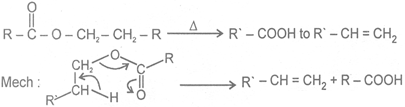
This reaction follows syn elimination & hoffman product is formes.
FAQs on Carboxylic Acids & Acids Derivatives, Class 12, Chemistry Detailed Chapter Notes
| 1. What are carboxylic acids and acid derivatives? |  |
| 2. How are carboxylic acids and acid derivatives named? |  |
| 3. What are the common uses of carboxylic acids and acid derivatives? |  |
| 4. How do carboxylic acids and acid derivatives react with nucleophiles? |  |
| 5. What are the differences between carboxylic acids and acid derivatives? |  |




















