Solved Subjective Problems : Biomolecules & Polymers | Organic Chemistry for NEET PDF Download
SOLVED Subjective PROBLEMS
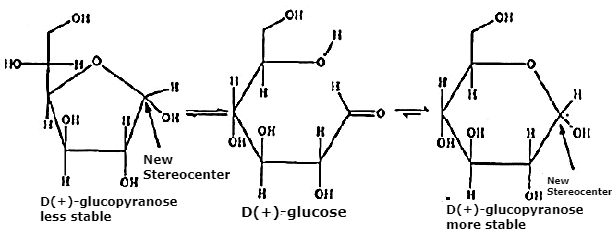
Problem 1: Write the hemiacetal formation for glucose.
Solution:
Problem 2: The pKa of the carboxyl group in an amino acid valine, (CH3)2CHCH(NH2)(COOH) is 2.31 and the pKa for the amino group of the same amino acid is 9.69. Compute the Isolectric point (pI) for valine and draw the structure of this amino acid when the pH of the solution equals to p1. Also draw the structures of valine that predominate at pH =2 and pOH =2.
Solution: The isoelectric point (pI) is the pH at which the amino acid exists only as a dipolar ion with net charge zero.
At isoelectric point, for a neutral amino acid, 
The dissociation of cationic form of valine can be represented as:
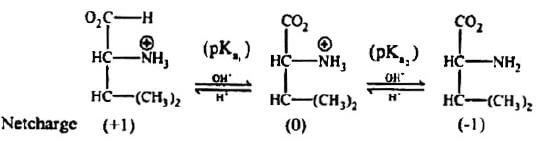
The species with zero net charge exists between species with ( +I) and (—I) net charges.
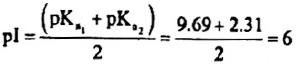
When the p1-I of the solution equals to p1, the structure of valine is 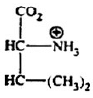
When the pH of the solution is two, the structure of valine is 
When the pH of the solution is 12, the structure of valine is 
Problem 3: Sucrose on hydrolysis yields a mixture which is
(a) Optically inactive (b) Dextrorotatory (c) Laevorotatory (d) Racemic
Solution: (c) Sucrose on hydrolysis yields equimolar mixture of D-(—)-fructose and D-(+)glucose. Since specific rotation of D(—)-fructose is greater than (+)-glucose D, the mixture is laevorotatory.
Problem 4: A high molecular weight molecule which does not contain repeating structural units is called a
(a) Polymer (b) Macromolecule (c) Both (a) and (b) (d) None of these
Solution: (b) A polymer has always repeating structural units derived from monomer. For example proteins and nucleic acid are regarded as macromolecules, but not polymers because their molecules do not contain repeating structural units. All polymers are macromolecules, but all macromolecules are not polymers.
Problem 5: The force of attraction between the neighbouring peptide chains is
(a) van der Waal's force (b) Covalent bond (c) Hydrogen bond (d) Peptide linkage
Solution: (c) Neighbouring peptide chains are held by hydrogen bonds between —CO— and — NH—.
Problem 6: Peptides on hydrolysis give
(a) Ammonia (b) Amines (c) Amino acids (d) Hydroxy acids
Solution: (c) Peptides are formed by condensation of a-amino acids. Therefore, on hydrolysis they yield a-amino acids.
Problem 7: An example of a condensation polymer is
(a) PVC (b) terylene (c) polypropylene (d) polystyrene
Solution: (b) In condensation polymerization, a series of condensation reactions between the (generally two) monomers containing atleast two functiønal groups each occur with the loss of a small molecule such as H2O, CH3OH or HX (X = halogen). Terylene is a condensation polymer of ethylene glycol and terephthalic acid.
Problem 8: Although both polymers are prepared by free radical processes, poly (vinyl chloride) is amorphous and poly (vinylidene chloride) (saran) is highly crystalline. How do you account for the different ? (vinylidene chloride is 1,1-dichloroethene).
Solution: As poly (vinyl chloride) is able to show stereoisomerism and further it is formed by a free radical process, it is atactic (chlorine atoms (distributed randomly), the molecules fit together poorly.
Poly (vinylidene chloride) has two identical substitutents on each carbon and the chains fit together well.
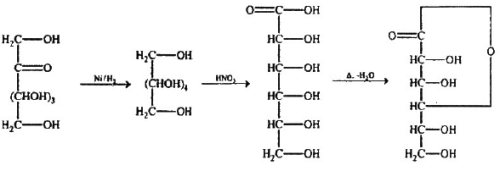
Problem 9: Compound A C5H10O4, is oxidized by Br2-H2O to the acid, C5H10O5. (A) Forms a triacetate (Ac2O) and is reduced by HI to n-pentane. Oxidation of (A) with HIO4 gives, among other product, 1 molecule of CH2O and 1 molecule of HCO2H. What are the possible strcutures of (A) and how could you distinguish between them ?
Solution: (A) is an aldehyde, contains three hydroxyl groups and the carbon skeleton consists of five carbon atoms in a straight chain. Also, the formula C5H10O4 therefore suggests that (A) is a deoxy-sugar. If we now try to work out the possibilities based directly on the periodic oxidation of (A), we shall find it.
Problem 10: Convert
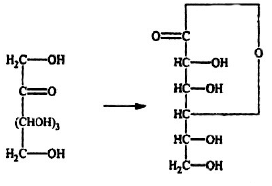
Solution:
Problem 11: i) Sulphanilic acid although has acidic as well as basic group, it is soluble in alkali but insoluble in mineral acid.
ii) Sulphanilic acid is not soluble in organic solvents.
Solution: i) Sulphanic acid exist as Zwitter ion.

The weakly acidic -NH3 transfers H to OH_ to form a soluble salt, P-NH2-C6H4-SO 3_Na on the other hand _SO3_ is too weakly basic to accept H from strong acids.
iii) Due to its ionic character it is insoluble in organic solvents.
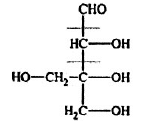
Problem 12: Compound (A) C5H10O5, give a tetra-acetate with Ac2O and oxidation of (A) with Br2 _ H2O gives an acid, C5H10O6. Reduction of (A) with HI and red phosphorous gives 3-methylbutane. What is structure of (A) ?
Solution: The formation of tetracetate indicates of 4OH group and oxidation with bromine water indicates presence of CHO group. Reduction with red phosphorous and HI indicates presence of one carbon in the side chain. Thus, the structure of (A) would be
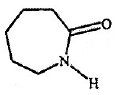
Problem 13: What is the structure of nylon-6, made by alkaline polymerisation of caprolactum ?
Solution: The configuration of these carbons which are unchanged in the reactions, must be identical in order to get the same osazone.
a) Nylon-6: Another polymer of this class is nylon-6. It is a monomer of caprolactum which is obtained from cyclohexane.

It used for making tyre cords, fabrics and ropes.
b) Nylon-6, 10: A polymer of hexamethylene diamine (six carbon atoms) and sebacic acid (ten carbon atoms).

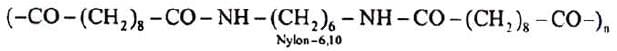
These polymers are formed by the condensation of two or more monomers with the elimination of simple molecules like H2O, NH3, ROH etc.
Problem 14: Supply structures for H through K. Given :
An Aldohexose 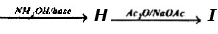
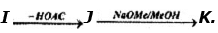
(b) Explain the last step (c). What is net structural change (d) Name this overall method. (c) Discuss the possibility of epimer formation.
Solution: a) H is an oxime HOCH2 (CHOH)4CH = NOH; is the completely acetylated oxime, AcOCH2(CHOH)4CH = NoAc that loses 1 mole of HOAc to form J, AcOCH2(CHOAc)4 C _ N; K is an aldopentose, HOCH2(CHOH)3CHO.
b) The acetates undergo trans-esterification to give methyl acetate freeing all the sugar OH's. This is followed by reversal of HCN addition.
c) There is loss of one C from the carbon chain.
d) Wohl Degradation.
e) The -CHOH becomes the -CH = O without any configurational changes of the other chiral carbons. Thus, no epimers are formed.
Problem 15: Glycine exists as (H3N CH2COO_) while anthranilic acid (P - NH2 - C6H4 - COOH) does not exist as dipolar ion.
Solution: -COOH is too weakly acidic to transfer H to the weakly basic -NH2 attached to the electron withdrawing benzene ring. When attached to an aliphatic carbon, the -NH2 is sufficiently basic to accept H from -COOH group.
Problem 16: Why should isoelectric point for Aspartic acid (2.98) be so much lower than that of leucine ?
Solution: This may be explained by considering following ion equilibrium
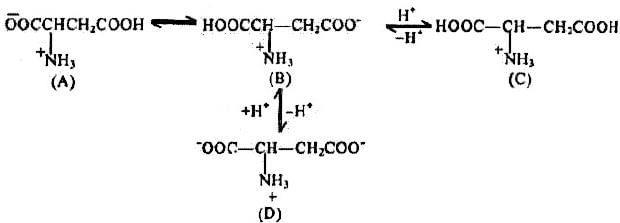
It is apparent that ions (A) and (B) are neutral, while (C) is a cation and (D) is dianion. In species (D), the anion is derived from the second -COOH group present in aspartic acid and is not possible in leucine. At neutral pH a significant concentration of (D), will be present in aqueous solution. It will therefore, be necessary to decrease the pH of such a solution if the formation of (D) is to be suppressed to a stage where anions and cations are present in equal concentration (the isoelectric point).
Problem 17: (a) Show how an aldohexose can be used to synthesize 2-ketohexose.
(b) Since glucose is converted to fructose by this method, what can you say about the configuration of C3, C4 and C5 in the sugars.
Solution:

Here aldohexose reacts with one molecule of phenylhydrazine which condenses with the aldehyde group to give phenylhydrazone. When warmed with excess of phenyl hydrazine, the secondary alcoholic group adjacent to the aldehyde group is oxidised by another molecule of phenylhydrazine, to a ketonic group. With this ketonic group, the third molecule of phenylhydrazine condenses to given osazone. The phenylhydrazinyl group is transferred from osazone to C6H5CHO giving C6H5CH = N.NHC6H5 and a dicarbonyl compound called Osone. The more reactive aldehyde group of the osone is reduced, not the less reactive keto group and it gives 2-ketohexose.
Problem 18: Starch is polymer of:
(a) Fructose (b) Glucose (c) Lactose (d) None
Solution: (b) Starch is homopolysaccharide of glucose having 24 — 30 glucose units.
Problem 19: The commonest disaccharide has the molecular formula
(a) C10H18O9 (b) C10H20O10 (c) C11H22O11 (d) C12H22O11
Solution: (d) The most common disaccharide is sucrose, whose molecular formula is C11H22O11.
Problem 20: The structure of glycine (amino acid) is H3N CH2 COO_ (Zwitter Ion.)
Select the correct statement of the following.
(a) Glycine, as well as other amino acids are amphoteric.
(b) The acidic functional group in amino acids is — NH3
(c) The basic functional group in amino acids is —CO2_
(d) All the statements are correct
Solution: (d) Glycine and all other amino acids are amphoteric because of the presence of NH2 and CO2H group both. The amino acid exists as Zwitter ion and acidic group is —NH3 while basic group is —CO2_
Problem 21: Sugars are characterised by the preparation of osazone derivatives. Which sugar have identical osazones.
(a) Glucose and lactose
(b) Glucose and fructose
(c) Glucose and arabinose
(d) Glucose and maltose
Solution: (b) The reaction with phenyl hydrazone gives same osazone because glucose and fructose differ only on carbon atoms 1 and 2 which are involved in osazone formation.
Problem 22: Cane sugar on hydrolysis yields
(a) Glucose and maltose
(b) Glucose and lactose
(c) Glucose and fructose
(d) Only glucose
Solution : (c) 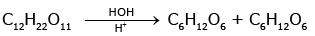
Glucose and Fructose
The process is known as inversion of cane sugar.
|
54 videos|125 docs|136 tests
|
FAQs on Solved Subjective Problems : Biomolecules & Polymers - Organic Chemistry for NEET
| 1. What are the different types of biomolecules? |  |
| 2. How are biomolecules formed? |  |
| 3. What are the functions of polymers in living organisms? |  |
| 4. How do biomolecules contribute to the structure and function of cells? |  |
| 5. What are the roles of biomolecules in disease and medicine? |  |






















